9.2 The Plasma Membrane
A cell’s plasma membrane separates the interior of the cell from its environment and determines how it interacts with the outside world. Cells exclude some substances, take in others, and excrete still others, all in controlled quantities. In addition, the plasma membrane’s surface carries markers that allow cells to recognize one another, which is vital for tissue and organ formation during early development, and which later plays a role in the immune response’s “self” versus “non-self” distinction.
Among the most sophisticated plasma membrane functions is the ability to transmit signals through protein receptors. These proteins act both as extracellular input receivers and as intracellular processing activators. These membrane receptors provide extracellular attachment sites for effectors like hormones and growth factors, which activate intracellular responses. Occasionally, viruses hijack receptors (HIV, human immunodeficiency virus, is one example) that use them to gain entry into cells, and at times, the genes encoding receptors become mutated, causing the signal transduction process to malfunction with disastrous consequences.
Fluid Mosaic Model
The fluid mosaic model describes the plasma membrane structure as a mosaic of components—including phospholipids, cholesterol, proteins, and carbohydrates—that gives the membrane a fluid character. Plasma membranes range from 5 to 10 nm in thickness. For comparison, human red blood cells, visible via light microscopy, are approximately 8 µm wide, or approximately 1,000 times wider than a plasma membrane.
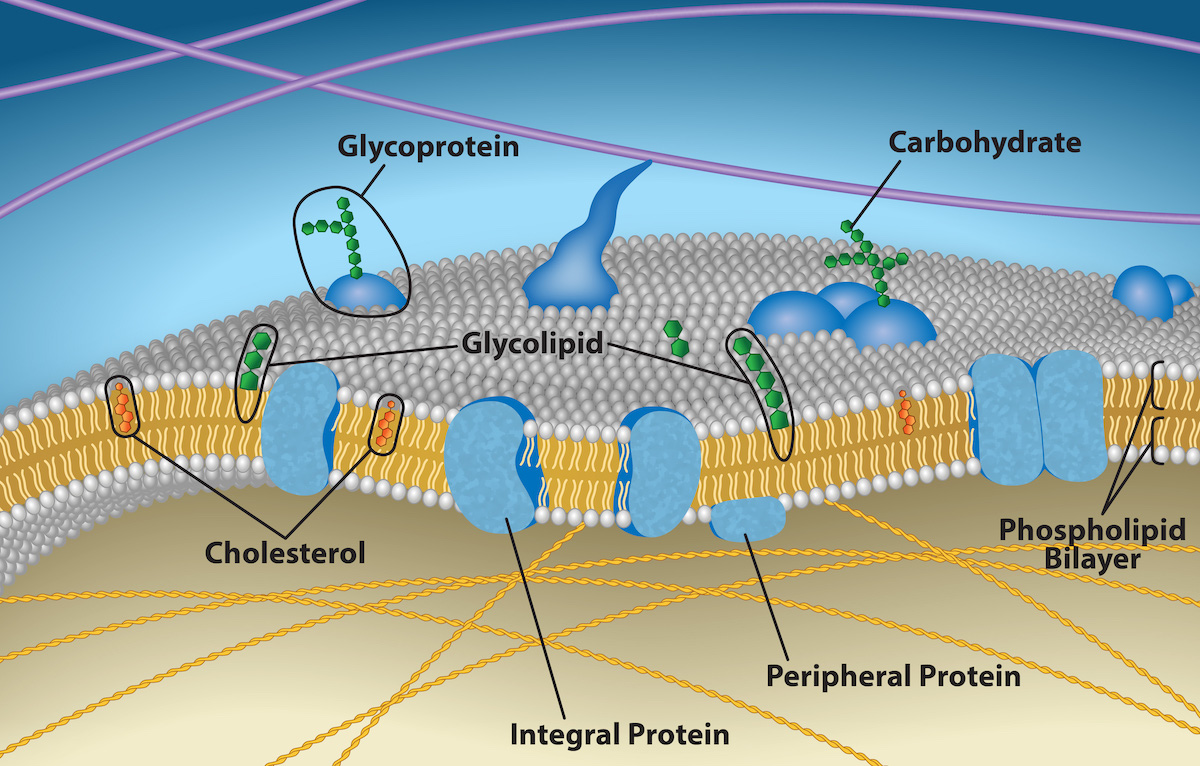
A plasma membrane’s principal components are lipids (phospholipids and cholesterol), proteins, and carbohydrates attached to some of the lipids and proteins. A phospholipid is a molecule consisting of glycerol, two fatty acids, and a phosphate-linked head group. Cholesterol, another lipid comprised of four fused carbon rings, is situated alongside the phospholipids in the membrane’s core. The protein, lipid, and carbohydrate proportions in the plasma membrane vary with cell type, but for a typical human cell, protein accounts for about 50 percent of the composition by mass, lipids (of all types) account for about 40 percent, and carbohydrates comprise the remaining 10 percent. However, protein and lipid concentration varies with different cell membranes. Carbohydrates are present only on the plasma membrane’s exterior surface and are attached to proteins, forming glycoproteins, or attached to lipids, forming glycolipids.
Phospholipids
The membrane’s main fabric is made of phospholipid molecules. The hydrophilic or “water-loving” areas of these molecules are in contact with the water both inside and outside the cell. Hydrophobic, or water-hating molecules, tend to be non-polar. They interact with other non-polar molecules in chemical reactions, but generally do not interact with polar molecules. When placed in water, hydrophobic molecules tend to form a ball or cluster.
The phospholipids’ hydrophilic regions form hydrogen bonds with water and other polar molecules on both the cell’s exterior and interior. Thus, the membrane surfaces that face the cell’s interior and exterior are hydrophilic. In contrast, the cell membrane’s interior is hydrophobic and will not interact with water. Therefore, phospholipids form an excellent two-layer cell membrane that separates fluid within the cell from the fluid outside the cell.
Proteins
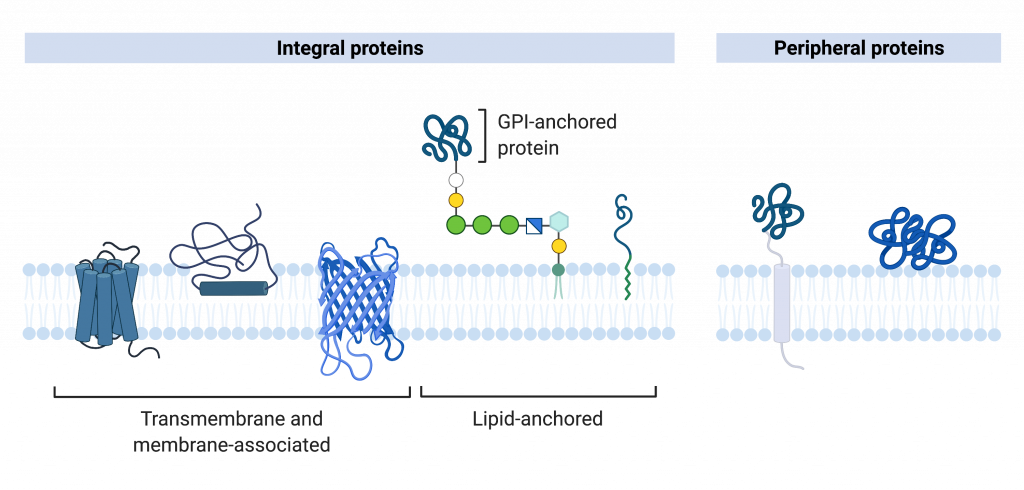
Proteins comprise the plasma membranes’ second major component. Integral proteins integrate completely into the membrane structure, and their hydrophobic membrane-spanning regions interact with the phospholipid bilayer’s hydrophobic region. Some integral membrane proteins belong to the category named transmembrane proteins because they stick out of the membrane on both sides. Single-pass transmembrane proteins usually have a hydrophobic transmembrane segment that consists of 20–25 amino acids. Other integral membrane proteins span only part of the membrane—associating with a single layer. Up to 12 single protein segments comprise some complex proteins, which are extensively folded and embedded in the membrane. This protein type has two or more hydrophilic regions, and one or several mildly hydrophobic regions. Such proteins are embedded in the membrane such that the hydrophobic regions of the protein are adjacent to the phosopholipid tails and the protein’s hydrophilic regions protrude from the membrane and are in contact with the cytosol or extracellular fluid.
Carbohydrates
Carbohydrates are the third major plasma membrane component. They are always on the cells’ exterior surface and are bound either to proteins (forming glycoproteins) or to lipids (forming glycolipids). These carbohydrate chains may consist of 2–60 monosaccharide units and can be either straight or branched. Along with peripheral proteins, carbohydrates form specialized sites on the cell surface that allow cells to recognize each other. These sites have unique patterns that allow for cell recognition, much the way that the facial features unique to each person allow individuals to recognize him or her. This recognition function is very important to cells, as it allows the immune system to differentiate between body cells (“self”) and foreign cells or tissues (“non-self”). Similar glycoprotein and glycolipid types are on the surfaces of viruses and may change frequently, preventing immune cells from recognizing and attacking them.
We collectively refer to these carbohydrates on the cell’s exterior surface—the carbohydrate components of both glycoproteins and glycolipids—as the glycocalyx (meaning “sugar coating”). The glycocalyx is highly hydrophilic and attracts large amounts of water to the cell’s surface. This aids in the cell’s interaction with its watery environment and in the cell’s ability to obtain substances dissolved in the water. The glycocalyx is also important for cell identification, self/non-self determination, and embryonic development, and is used in cell to cell attachments to form tissues.
Membrane Fluidity
The membrane’s mosaic characteristic helps to illustrate its nature. The integral proteins and lipids exist in the membrane as separate but loosely attached molecules. These resemble the separate, multicolored tiles of a mosaic picture, and they float, moving somewhat with respect to one another. The membrane is not like a balloon, however, that can expand and contract. It is fairly rigid and can burst if penetrated or if a cell takes in too much water. However, because of its mosaic nature, a very fine needle can penetrate a plasma membrane without causing it to burst, and the membrane will flow and self-seal when one extracts the needle.
The membrane’s mosaic characteristics explain some but not all of its fluidity. There are two other factors that help maintain this fluid characteristic. One factor is the nature of the phospholipids themselves. In their saturated form, the fatty acids in phospholipid tails are saturated with bound hydrogen atoms. There are no double bonds between adjacent carbon atoms. This results in tails that are relatively straight. In contrast, unsaturated fatty acids do not contain a maximal number of hydrogen atoms, but they do contain some double bonds between adjacent carbon atoms. A double bond results in a bend in the carbon string of approximately 30 degrees.
Thus, if decreasing temperatures compress saturated fatty acids with their straight tails, they press in on each other, increasing the opportunity for van der Waals interactions between them, and making a fairly rigid membrane. If unsaturated fatty acids are compressed, the “kinks” in their tails elbow adjacent phospholipid molecules away, maintaining some space between the phospholipid molecules, and reducing the opportunity for van der Waals attractions between them. This “elbow room” helps to maintain fluidity in the membrane at temperatures at which membranes with saturated fatty acid tails in their phospholipids would “freeze” or solidify. The membrane’s relative fluidity is particularly important in a cold environment. A cold environment usually compresses membranes comprised largely of saturated fatty acids, making them less fluid and more susceptible to rupturing. Many organisms (fish are one example) are capable of adapting to cold environments by changing the proportion of unsaturated fatty acids in their membranes in response to lower temperature.
Animals have an additional membrane constituent that assists in maintaining fluidity. Cholesterol, which lies alongside the phospholipids in the membrane, tends to dampen temperature effects on the membrane. Thus, this lipid functions as a buffer, preventing lower temperatures from inhibiting fluidity and preventing increased temperatures from increasing fluidity too much. Thus, cholesterol extends, in both directions, the temperature range in which the membrane is appropriately fluid and consequently functional. Cholesterol also serves other functions, such as organizing clusters of transmembrane proteins into lipid rafts.
protein in or on a target cell that binds to ligands, resulting in a response
a signaling molecule that regulates physiology, behavior, or development
biological macromolecule in which the ratio of carbon to hydrogen and to oxygen is 1:2:1; serve as energy sources and structural support molecules
protein that has carbohydrates covalently bound to it
lipid that has carbohydrates covalently bound to it
the most common steroid; precursor to hormones such as testosterone and estradiol; also found in the plasma membrane of animal cells

