4.4 Non-covalent bonds
Ionic Bonds
Some atoms are more stable when they gain or lose an electron (or possibly two) and form ions. This fills their outermost electron shell and makes them energetically more stable. Because the number of electrons does not equal the number of protons, each ion has a net charge. Cations are positive ions that form by losing electrons. Negative ions form by gaining electrons, which we call anions. We designate anions by their elemental name and change the ending to “-ide”, thus the anion of chlorine is chloride, and the anion of sulfur is sulfide.
Ions can be formed by electron transfer. For example, sodium (Na) only has one electron in its outer electron shell. It takes less energy for sodium to donate that one electron than it does to accept seven more electrons to fill the outer shell. If sodium loses an electron, it now has 11 protons, 11 neutrons, and only 10 electrons, leaving it with an overall charge of +1. We now refer to it as a sodium ion. Chlorine (Cl) has seven electrons in its outer shell. Again, it is more energy-efficient for chlorine to gain one electron than to lose seven. Therefore, it tends to gain an electron to create an ion with 17 protons, 17 neutrons, and 18 electrons, giving it a net negative (–1) charge. We now refer to it as a chloride ion.
In this example, sodium will donate its one electron to empty its shell, and chlorine will accept that electron to fill its shell. Both ions now have complete outermost shells. Because the number of electrons is no longer equal to the number of protons, each is now an ion and has a +1 (sodium cation) or –1 (chloride anion) charge. Note that these transactions can normally only take place simultaneously: in order for a sodium atom to lose an electron, it must be in the presence of a suitable recipient like a chlorine atom.
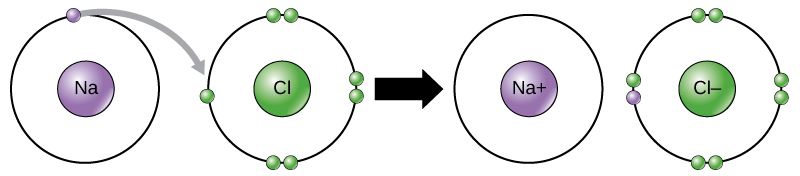
Ionic bonds form between ions with opposite charges. For instance, positively charged sodium ions and negatively charged chloride ions bond together to make crystals of sodium chloride, or table salt, creating a crystalline molecule with zero net charge.
Hydrogen Bonds
When polar covalent bonds containing hydrogen form, the hydrogen in that bond has a slightly positive charge because hydrogen’s electron is pulled more strongly toward the other element and away from the hydrogen. Because the hydrogen is slightly positive, it will be attracted to neighboring negative charges. When this happens, a weak interaction occurs between the hydrogen’s δ+ from one molecule and the molecule’s δ– charge on another molecule with the more electronegative atoms, usually oxygen. Scientists call this interaction a hydrogen bond.
This type of bond is common and occurs regularly between water molecules. Individual hydrogen bonds are weak and easily broken; however, they occur in very large numbers in water and in organic polymers, creating a major force in combination. Hydrogen bonds are also responsible for zipping together the DNA double helix.
Van Der Waals Interactions
Like hydrogen bonds, van der Waals interactions are weak attractions or interactions between molecules. Van der Waals attractions can occur between any two or more molecules and are dependent on slight fluctuations of the electron densities, which are not always symmetrical around an atom. For these attractions to happen, the molecules need to be very close to one another. These bonds—along with ionic, covalent, and hydrogen bonds—contribute to the proteins’ three-dimensional structure in our cells that is necessary for their proper function.
atom, molecule, or chemical group that does not contain equal numbers of protons and electrons and is therefore charged
positive ion that is formed by an atom losing one or more electrons
negative ion that is formed by an atom gaining one or more electrons
movement of electrons from one element to another
chemical bond that forms between ions with opposite charges
weak bond between slightly positively charged hydrogen atoms and slightly negatively charged atoms in other molecules
very weak interaction between molecules due to temporary charges attracting atoms that are very close together

