22.3 Electron Transport Chains
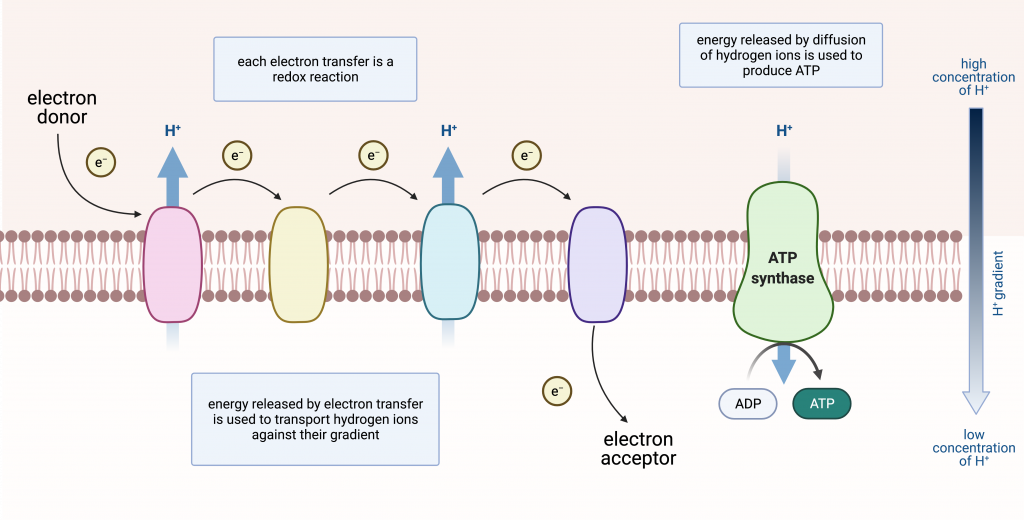
You now know that ATP is generated by using the energy released when hydrogen ions flow down their gradient. How is the hydrogen ion gradient established and maintained? The answer is by an electron transport chain, in which the energy from electrons is used to pump hydrogen ions against their gradient, establishing a region of high concentration. This gradient is a source of potential energy, which is then used to produce ATP.
Redox Reactions
Electron transport chains rely on the transfer of electrons. Electron transfer involves oxidation and reduction reactions, which occur at the same time. An oxidation reaction strips an electron from an atom in a compound, and the addition of this electron to another compound is a reduction reaction. Because oxidation and reduction usually occur together, these pairs of reactions are called oxidation reduction reactions, or redox reactions.
Many molecules can exist in a reduced form or an oxidized form. The reduced form has more free energy than the oxidized form.

Electrons and Energy
The removal of an electron from a molecule (oxidizing it), results in a decrease in potential energy in the oxidized compound. The electron is shifted to a second compound, reducing the second compound. The shift of an electron from one compound to another removes some potential energy from the first compound (the compound being oxidized) and increases the potential energy of the second compound (the compound being reduced). The transfer of electrons between molecules is important because most of the energy stored in atoms and used to fuel cell functions is in the form of high-energy electrons. The transfer of energy in the form of high-energy electrons allows the cell to transfer and use energy in an incremental fashion—in small packages rather than in a single, destructive burst.
Electron Transport Chains
Electron transport chains involve electrons being passed from one molecule to another. The major components are protein complexes embedded in a membrane (inner mitochondrial membrane, chloroplast thylakoid membrane, or prokaryotic plasma membrane). Electron transport is a series of redox reactions that resembles a relay race or bucket brigade in that electrons are passed rapidly from one component to the next, to the endpoint of the chain. The flow of electrons is exergonic. Some of the energy released can be used to do work, specifically pumping hydrogen ions against their gradient. This is what maintains the gradient that drives ATP production.
energy type that has the potential to do work; stored energy
loss of electrons
gain of electrons
reaction in which an electron is transferred between two substances

