10.2 Osmosis
Osmosis is the diffusion of water through a semipermeable membrane. While diffusion transports material across membranes and within cells, osmosis transports only water across a membrane and the membrane limits the solutes’ diffusion in the water. Not surprisingly, the aquaporins that facilitate water movement play a large role in osmosis.
Mechanism
Osmosis is a special case of diffusion. Water, like other substances, moves from an area of high concentration of free water molecules to one of low free water molecule concentration. An obvious question is what makes water move at all? Imagine a beaker with a semipermeable membrane separating the two sides or halves. On both sides of the membrane the water level is the same, but there are different concentrations of a dissolved substance, or solute, that cannot cross the membrane (otherwise the solute crossing the membrane would balance concentrations on each side). If the solution’s volume on both sides of the membrane is the same, but the concentrations of the solute are different, then the concentrations of the water, the solvent, will also be different on either side of the membrane.
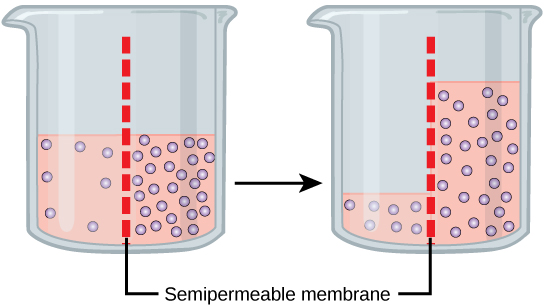
A principle of diffusion is that the molecules move around and will spread evenly throughout the medium if they can. However, only the material capable of getting through the membrane will diffuse through it. In this example, the solute cannot diffuse through the membrane, but the water can. Water has a concentration gradient in this system. Thus, water will diffuse down its concentration gradient, crossing the membrane to the side where it is less concentrated. This diffusion of water through the membrane—osmosis—will continue until the water’s concentration gradient goes to zero or until the water’s hydrostatic pressure balances the osmotic pressure. Osmosis takes place constantly in living systems.
Tonicity
Tonicity describes how an extracellular solution can change a cell’s volume by affecting osmosis. A solution’s tonicity often directly correlates with the solution’s osmolarity. Osmolarity describes the solution’s total solute concentration.
Hypotonic Solutions
Scientists use three terms—hypotonic, isotonic, and hypertonic—to relate the cell’s osmolarity to the extracellular fluid’s osmolarity that contains the cells. In a hypotonic situation, the extracellular fluid has lower osmolarity than the fluid inside the cell, and water enters the cell. (In living systems, the point of reference is always the cytoplasm, so the prefix hypo– means that the extracellular fluid has a lower solute concentration, or a lower osmolarity, than the cell cytoplasm.) It also means that the extracellular fluid has a higher water concentration in the solution than does the cell. In this situation, water will follow its concentration gradient and enter the cell.
Hypertonic Solutions
A hypertonic solution refers to a solution having a higher solute concentration or osmolarity than the cell’s cytoplasm; therefore, the extracellular fluid contains a lower concentration of water than the cell does. Because the cell has a relatively higher water concentration, water will leave the cell.
Isotonic Solutions
An isotonic solution has the same osmolarity as the cell. Since the cell’s osmolarity matches that of the extracellular fluid, there will be no net movement of water into or out of the cell, although water will still move in and out. Blood cells and plant cells in hypertonic, isotonic, and hypotonic solutions take on characteristic appearances.
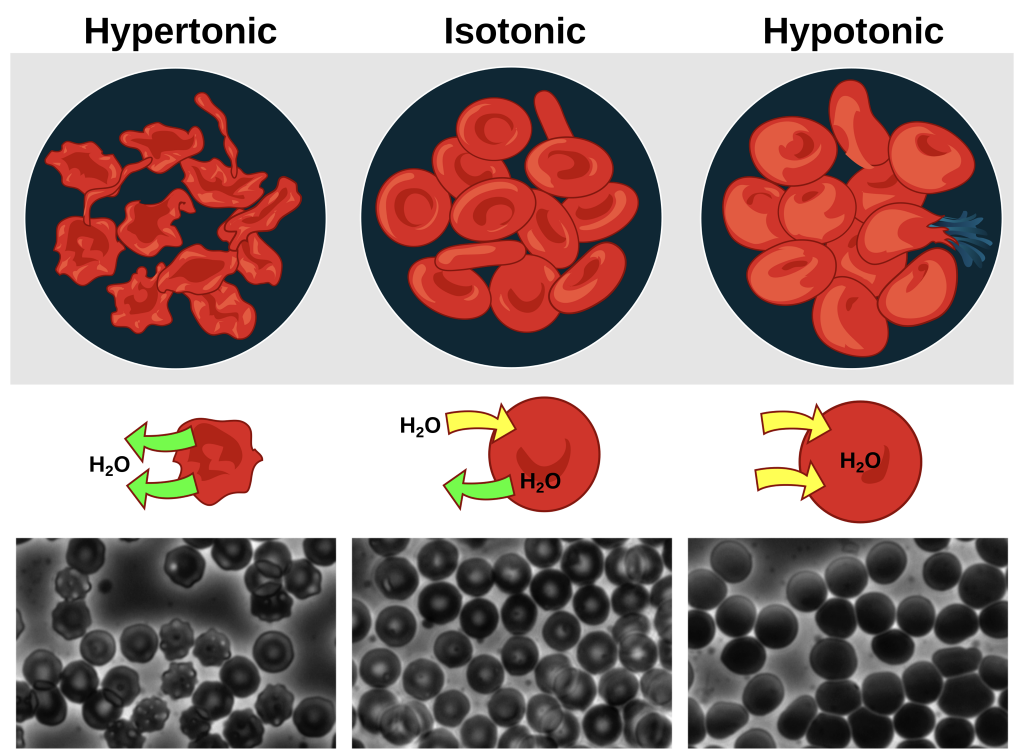
Tonicity in Living Systems
In a hypotonic environment, water enters a cell, and the cell swells. In an isotonic condition, the relative solute and solvent concentrations are equal on both sides of the membrane. There is no net direction of water movement; therefore, there is no change in the cell’s size. In a hypertonic solution, water leaves a cell and the cell shrinks. If either the hypo- or hyper- condition goes to excess, the cell’s functions become compromised, and the cell may be destroyed.
A red blood cell will burst, or lyse, when it swells beyond the plasma membrane’s capability to expand.
In contrast, when excessive water amounts leave a red blood cell, the cell shrinks. This results in an increase of solute concentration in the cell, making the cytosol denser and interfering with diffusion within the cell. The cell’s ability to function will be compromised and may result in the cell’s death.
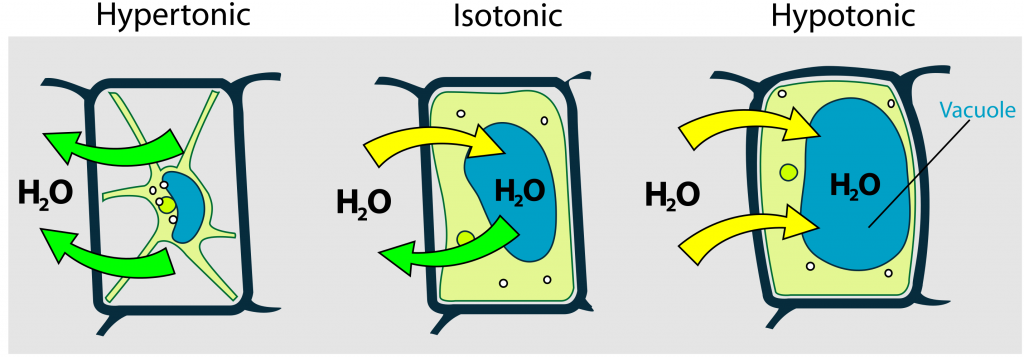
Various living things have ways of controlling the effects of osmosis—a mechanism we call osmoregulation. Some organisms, such as plants, fungi, bacteria, and some protists, have cell walls that surround the plasma membrane and prevent cell lysis in a hypotonic solution. The plasma membrane can only expand to the cell wall’s limit, so the cell will not lyse.
The cytoplasm in plants is always slightly hypertonic with respect to the extracellular environment, and water will always enter a cell if water is available. This water inflow produces turgor pressure, which stiffens the plant’s cell walls. In nonwoody plants, turgor pressure supports the plant. Conversely, if you do not water the plant, the extracellular fluid will become hypertonic, causing water to leave the cell. In this condition, the cell does not shrink because the cell wall is not flexible. However, the cell membrane detaches from the wall and constricts the cytoplasm. Plants lose turgor pressure in this condition and wilt.
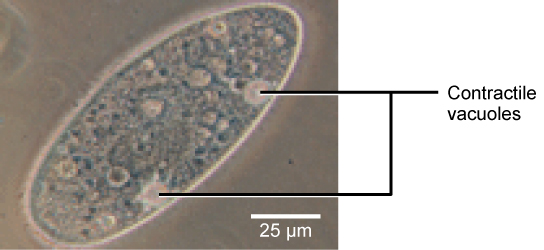
Tonicity is a concern for all living things. For example, paramecia and amoebas, which are protists that lack cell walls, have contractile vacuoles. These vaculoes collect excess water from the cell and pumps it out, keeping the cell from lysing as it takes on water from its environment.
Many marine invertebrates have internal salt levels matched to their environments, making them isotonic with the water in which they live. Fish, however, must spend approximately five percent of their metabolic energy maintaining osmotic homeostasis. Freshwater fish live in an environment that is hypotonic to their cells. These fish actively take in salt through their gills and excrete diluted urine to rid themselves of excess water. Saltwater fish live in the reverse environment, which is hypertonic to their cells, and they secrete salt through their gills and excrete highly concentrated urine.
transport of water through a semipermeable membrane according to the water's concentration gradient across the membrane that results from the presence of solute that cannot pass through the membrane
channel protein that allows water through the membrane at a very high rate
a substance dissolved in another substance
substance capable of dissolving another substance
amount of solute in a solution
total amount of solutes dissolved in a specific amount of solution
situation in which extracellular fluid has a lower osmolarity than the fluid inside the cell, resulting in water moving into the cell
situation in which extracellular fluid has a higher osmolarity than the fluid inside the cell, resulting in water moving out of the cell
situation in which the extracellular fluid has the same osmolarity as the fluid inside the cell, resulting in no net water movement into or out of the cell

