5.4 pH, Acids, and Bases
Dissociation of Water
Hydrogen ions spontaneously generate in pure water by the dissociation of a small percentage of water molecules into equal numbers of hydrogen (H+) ions and hydroxide (OH–) ions. While the hydroxide ions are kept in solution by their hydrogen bonding with other water molecules, the hydrogen ions, consisting of naked protons, immediately attract to un-ionized water molecules, forming hydronium ions (H3O+). Still, by convention, scientists refer to hydrogen ions and their concentration as if they were free in this state in liquid water.
2 H2O ↔ H3O+ + OH–
Two water molecules dissociate into a hydronium ion and a hydroxide ion
H2O ↔ H+ + OH–
We simplify to this chemical reaction and consider H+ ions to calculate pH
The concentration of hydrogen ions dissociating from pure water is 1 × 10-7 moles[1] H+ ions per liter of water. We calculate the pH as the negative of the base 10 logarithm of this concentration. The log10 of 1 × 10-7 is -7.0, and the negative of this number (indicated by the “p” of “pH”) yields a pH of 7.0, which is also a neutral pH. The pH inside of human cells and blood are examples of two body areas where near-neutral pH is maintained.
pH = -log[H+]
This is the equation to calculate pH, where [H+] is the molar concentration of hydrogen ions
Acids and Bases
Non-neutral pH readings result from dissolving acids or bases in water. High concentrations of hydrogen ions yield a low pH number, and low levels of hydrogen ions result in a high pH. An acid is a substance that increases hydrogen ions’ (H+) concentration in a solution, usually by having one of its hydrogen atoms dissociate. A base provides either hydroxide ions (OH–) or other negatively charged ions that combine with hydrogen ions, reducing their concentration in the solution and thereby raising the pH. In cases where the base releases hydroxide ions, these ions bind to free hydrogen ions, generating new water molecules.
The stronger the acid, the more readily it donates H+. For example, hydrochloric acid (HCl) completely dissociates into hydrogen and chloride ions and is highly acidic; whereas the acids in tomato juice or vinegar do not completely dissociate and are weak acids. Conversely, strong bases are those substances that readily donate OH– or take up hydrogen ions. Sodium hydroxide (NaOH) and many household cleaners are highly alkaline and give up OH– rapidly when we place them in water, thereby raising the pH. An example of a weak basic solution is seawater, which has a pH near 8.0 This is close enough to a neutral pH that marine organisms have adapted in order to live and thrive in a saline environment.
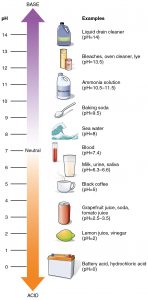
The pH Scale
The pH scale is, as we previously mentioned, an inverse logarithm and ranges from 0 to 14. Anything below 7.0 (ranging from 0.0 to 6.9) is acidic, and anything above 7.0 (from 7.1 to 14.0) is alkaline or basic. Extremes in pH in either direction from 7.0 are usually inhospitable to life. The pH inside cells (6.8) and the pH in the blood (7.4) are both very close to neutral. However, the environment in the stomach is highly acidic, with a pH of 1 to 2. As a result, how do stomach cells survive in such an acidic environment? How do they homeostatically maintain the near neutral pH inside them? The answer is that they cannot do it and are constantly dying. The stomach constantly produces new cells to replace dead ones, which stomach acids digest. Scientists estimate that the human body completely replaces the stomach lining every seven to ten days.
Buffers
How can organisms whose bodies require a near-neutral pH ingest acidic and basic substances (a human drinking orange juice, for example) and survive? Buffers are the key. Buffers readily absorb excess H+ or OH–, keeping the body’s pH carefully maintained in the narrow range required for survival. Maintaining a constant blood pH is critical to a person’s well-being. The buffer maintaining the pH of human blood involves carbonic acid (H2CO3), bicarbonate ion (HCO3–), and carbon dioxide (CO2). When bicarbonate ions combine with free hydrogen ions and become carbonic acid, it removes hydrogen ions and moderates pH changes. Similarly, excess carbonic acid can convert to carbon dioxide gas which we exhale through the lungs. This prevents too many free hydrogen ions from building up in the blood and dangerously reducing the blood’s pH. Likewise, if too much OH– enters into the system, carbonic acid will combine with it to create bicarbonate, lowering the pH. Without this buffer system, the body’s pH would fluctuate enough to put survival in jeopardy.
H+ + HCO3– ↔ H2CO3 ↔ H2O + CO2
How the body buffers blood pH via the bicarbonate system
Other examples of buffers are antacids that some people use to combat excess stomach acid. Many of these over-the-counter medications work in the same way as blood buffers, usually with at least one ion capable of absorbing hydrogen and moderating pH, bringing relief to those who suffer “heartburn” after eating. Water’s unique properties that contribute to this capacity to balance pH—as well as water’s other characteristics—are essential to sustaining life on Earth.
- Moles (mol) are a way to express the amount of a substance (which can be atoms, molecules, ions, etc.). One mole represents the atomic weight of a substance, expressed in grams, which equals the amount of the substance containing as many units as there are atoms in 12 grams of 12C. Mathematically, one mole is equal to 6.02 × 1023 particles of the substance. Therefore, 1 mole of water is equal to 6.02 × 1023 water molecules. ↵
separation of ions
molecule that donates hydrogen ions and increases the concentration of hydrogen ions in a solution
molecule that donates hydroxide ions or otherwise binds excess hydrogen ions and decreases the hydrogen ions' concentration in a solution
scale ranging from zero to 14 that is inversely proportional to the hydrogen ions' concentration in a solution
ability of an organism to maintain constant internal conditions
substance that resists a change in pH by absorbing or releasing hydrogen or hydroxide ions

