24.2 Light Energy
What Is Light Energy?
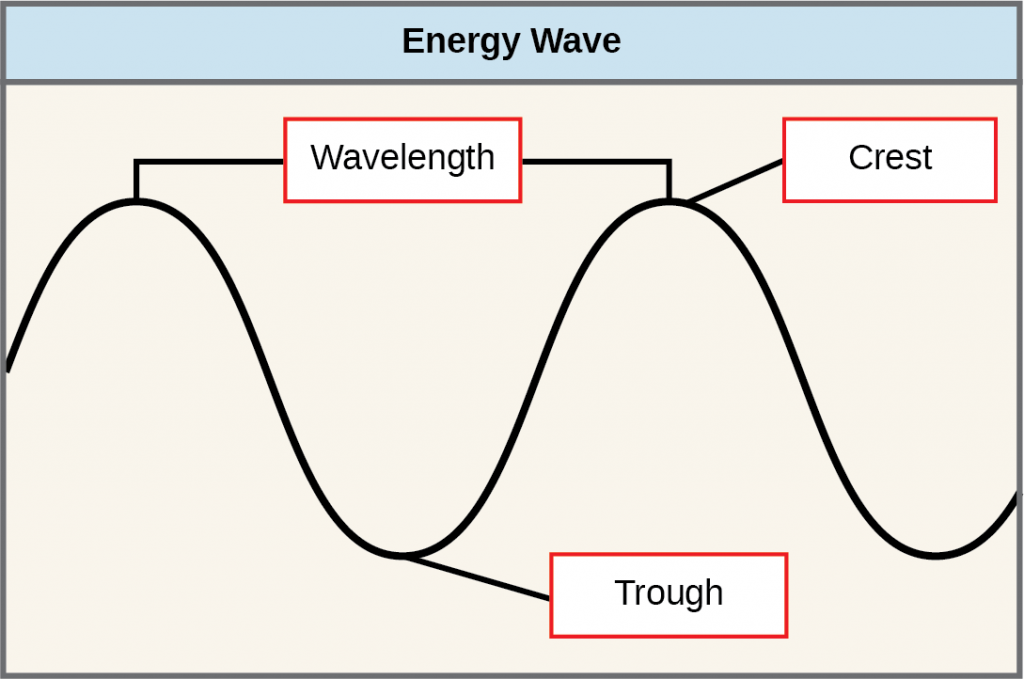
The sun emits an enormous amount of electromagnetic radiation (solar energy in a spectrum from very short gamma rays to very long radio waves). Humans can see only a tiny fraction of this energy, which we refer to as “visible light.” The manner in which solar energy travels is described as waves. Scientists can determine the amount of energy of a wave by measuring its wavelength (shorter wavelengths are more powerful than longer wavelengths)—the distance between consecutive crest points of a wave.
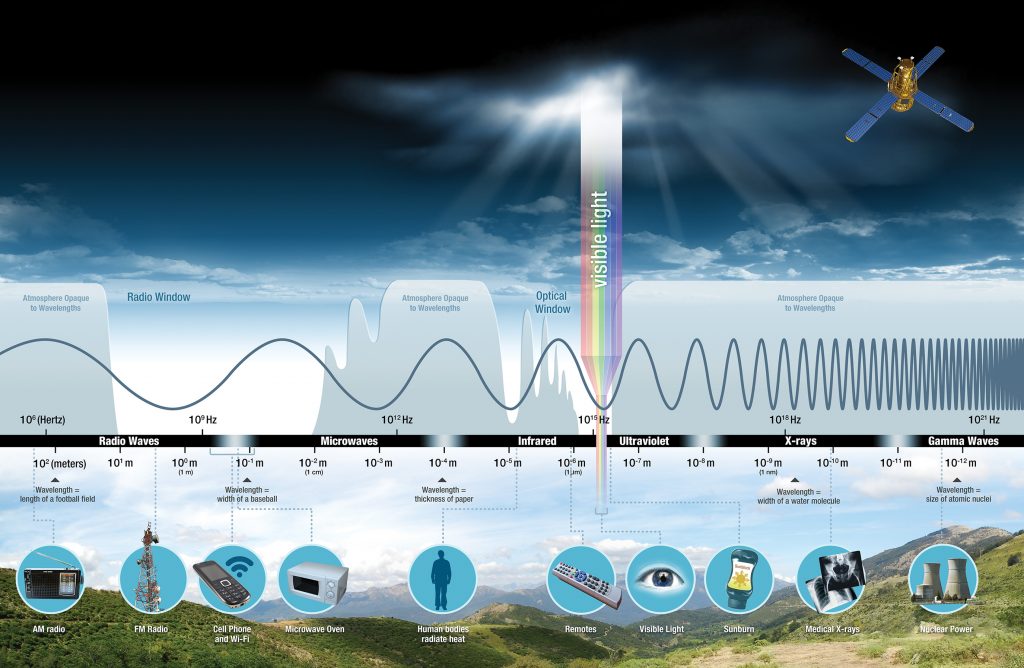
Visible light constitutes only one of many types of electromagnetic radiation emitted from the sun and other stars. Scientists differentiate the various types of radiant energy from the sun within the electromagnetic spectrum. The electromagnetic spectrum is the range of all possible frequencies of radiation.
Absorption of Light
Light energy initiates the process of photosynthesis when pigments absorb specific wavelengths of visible light. Organic pigments, whether in the human retina or the chloroplast thylakoid, have a narrow range of energy levels that they can absorb. Energy levels lower than those represented by red light are insufficient to raise an orbital electron to a excited (quantum) state. Energy levels higher than those in blue light will physically tear the molecules apart, in a process called bleaching. Our retinal pigments can only “see” (absorb) wavelengths between 700 nm and 400 nm of light, a spectrum that is therefore called visible light. In plants, pigment molecules only absorb those wavelengths of light in the range of 700 nm to 400 nm; therefore, plant physiologists refer to this range for plants as photosynthetically active radiation.
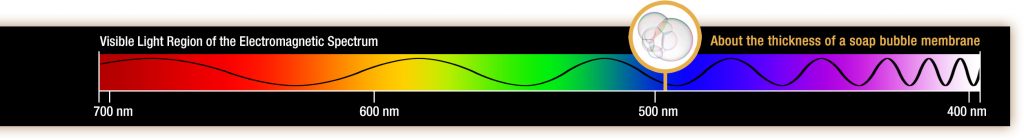
The visible light portion of the electromagnetic spectrum shows the rainbow of colors, with violet and blue having shorter wavelengths, and therefore higher energy. At the other end of the spectrum toward red, the wavelengths are longer and have lower energy.
Pigments
Different kinds of pigments exist, and each absorbs only specific wavelengths (colors) of visible light. Pigments reflect or transmit the wavelengths they cannot absorb; the color we see results from the mixture of reflected or transmitted light. Hence a green leaf reflects green light and absorbs purple, blue, red, and orange light.
Chlorophylls and carotenoids are the two major classes of photosynthetic pigments found in plants and algae; each class has multiple types of pigment molecules. There are five major chlorophylls, but only two are found in plant cells, chlorophyll a and chlorophyll b.
With dozens of different forms, carotenoids are a much more diverse group of pigments. The carotenoids found in fruit—such as the red of tomato (lycopene), the yellow of corn seeds (zeaxanthin), or the orange of an orange peel (β-carotene)—are used as advertisements to attract seed dispersers. In photosynthesis, carotenoids function as photosynthetic pigments that are very efficient molecules for the disposal of excess energy. When a leaf is exposed to full sun, the light-dependent reactions are required to process an enormous amount of energy; if that energy is not handled properly, it can do significant damage. Therefore, many carotenoids reside in the thylakoid membrane, absorb excess energy, and safely dissipate that energy as heat. Carotenoids can also absorb light energy that contributes to photosynthesis.
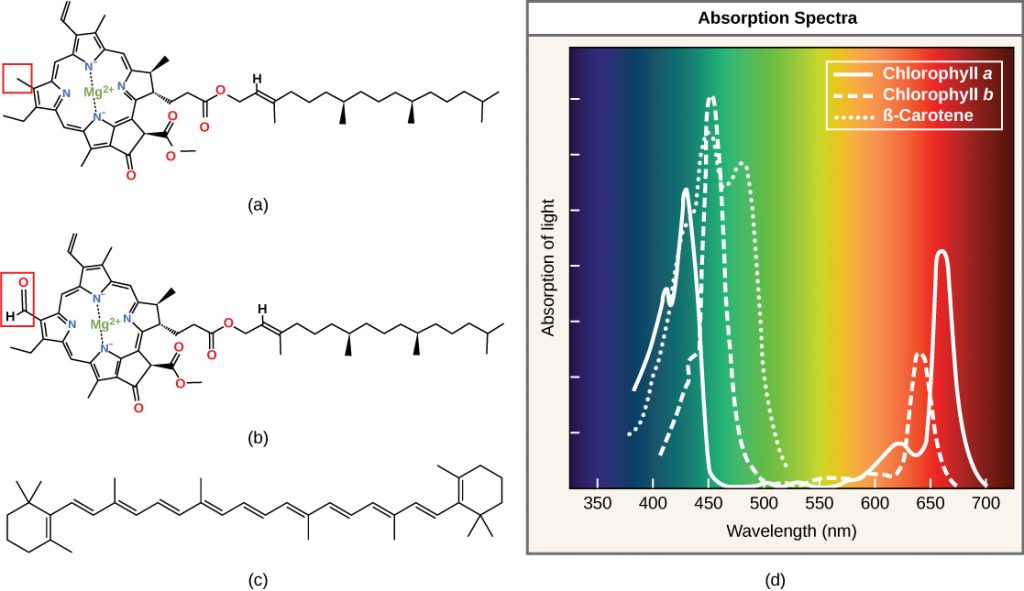
Each type of pigment can be identified by the specific pattern of wavelengths it absorbs from visible light: This is termed the absorption spectrum. The graph shows the absorption spectra for chlorophyll a, chlorophyll b, and a type of carotenoid pigment called β-carotene (which absorbs blue and green light). Notice how each pigment has a distinct set of peaks and troughs, revealing a highly specific pattern of absorption. Chlorophyll a absorbs wavelengths from either end of the visible spectrum (blue and red), but not green. Because green is reflected or transmitted, chlorophyll appears green. Carotenoids absorb in the short-wavelength blue region, and reflect the longer yellow, red, and orange wavelengths.
Many photosynthetic organisms have a mixture of pigments, and by using these pigments, the organism can absorb energy from a wider range of wavelengths. Not all photosynthetic organisms have full access to sunlight. Some organisms grow underwater where light intensity and quality decrease and change with depth. Other organisms grow in competition for light. Plants on the rainforest floor must be able to absorb any bit of light that comes through, because the taller trees absorb most of the sunlight and scatter the remaining solar radiation.
range of all possible frequencies of radiation
molecule that is capable of absorbing certain wavelengths of light and reflecting others (which accounts for its color)
form of chlorophyll that absorbs violet-blue and red light and consequently has a bluish-green color
accessory pigment that absorbs blue and red-orange light and consequently has a yellowish-green tint
photosynthetic pigment (yellow-orange-red) that functions to dispose of excess energy

