7.4 Protein Folding, Regulation, and Denaturation
Protein Folding
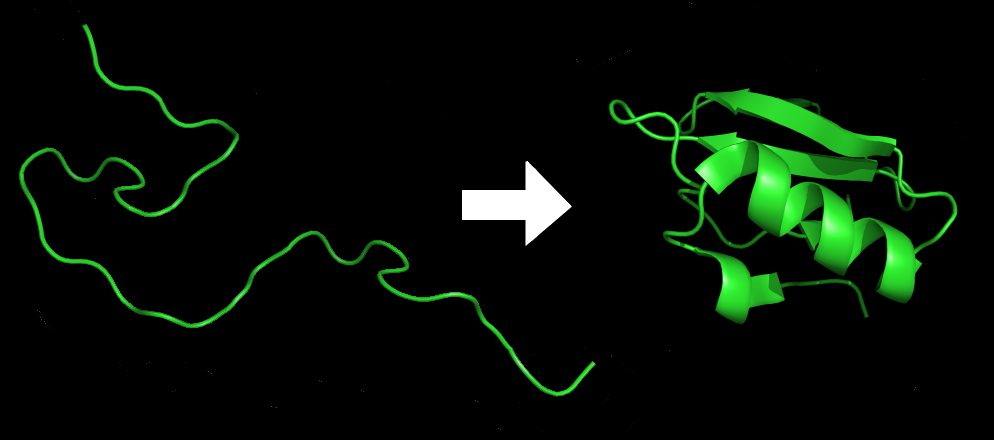
Protein folding is critical to its function. Scientists originally thought that the proteins themselves were responsible for the folding process. Only recently researchers discovered that often they receive assistance in the folding process from protein helpers, chaperonins, that associate with the target protein during the folding process. They act by preventing polypeptide aggregation that comprise the complete protein structure, and they disassociate from the protein once the target protein is folded.
Protein Regulation
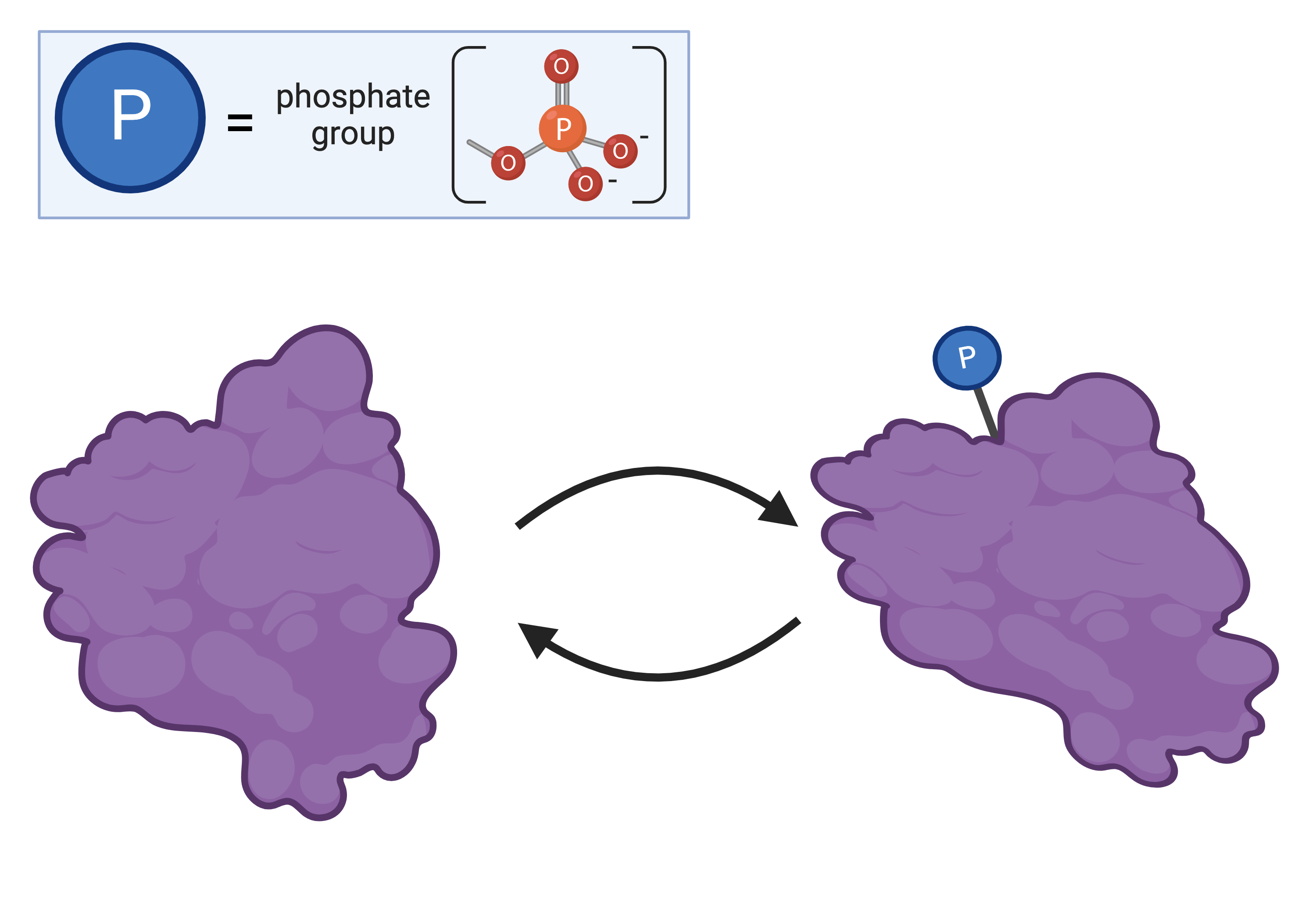
Proteins can be regulated by molecules that bind to them, which change the protein’s three-dimensional shape. For example, many proteins are regulated by adding phosphate groups (phosphorylation) or removing phosphate groups (dephosphorylation).
Denaturation
Each protein has its own unique sequence and shape that chemical interactions hold together. Changes in temperature, pH, or exposure to chemicals, may change the protein structure. The protein loses its shape without losing its primary sequence in what scientists call denaturation. Denaturation is sometimes reversible, allowing the protein to refold and resume its function. Sometimes denaturation is irreversible, leading to loss of function. One example of irreversible protein denaturation is frying an egg. The albumin protein in the liquid egg white denatures when placed in a hot pan. Not all proteins denature at high temperatures. For instance, bacteria that survive in hot springs have proteins that function at temperatures close to boiling. The stomach is also very acidic, has a low pH, and denatures proteins as part of the digestion process; however, the stomach’s digestive enzymes retain their activity under these conditions.
protein that helps a newly made protein in the folding process
addition of a phosphate group
removal of a phosphate group
loss of shape in a protein; can happen as a result of changes in temperature, pH, or chemical exposure

