4.2 The Structure of Atoms
Atomic Structure
An atom is the smallest unit of matter that retains all of the element’s chemical properties. For example, one gold atom has all of the properties of gold in that it is a solid metal at room temperature. A gold coin is simply a very large number of gold atoms molded into the shape of a coin and contains small amounts of other elements known as impurities. We cannot break down gold atoms into anything smaller while still retaining the properties of gold.
An atom is composed of two regions: the atomic nucleus, which is in the atom’s center and contains protons and neutrons. The atom’s outermost region holds its electrons in orbit around the nucleus. Atoms contain protons, electrons, and neutrons. The only exception is hydrogen (H), which is generally made of one proton and one electron with no neutrons.
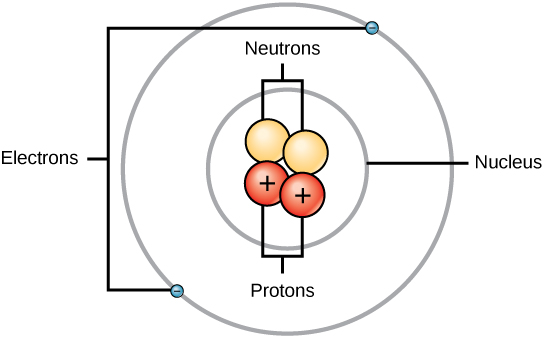
Atomic Number
Atoms of each element contain a characteristic number of protons and electrons. The number of protons determines an element’s atomic number, which scientists use to distinguish one element from another. The number of neutrons is variable, resulting in isotopes, which are different forms of the same atom that vary only in the number of neutrons they possess.
Electron Shells and the Bohr Model
In all electrically neutral atoms, the number of electrons is the same as the number of protons. Thus, each element, at least when electrically neutral, has a characteristic number of electrons equal to its atomic number.
In 1913, Danish scientist Niels Bohr (1885–1962) developed an early model of the atom. The Bohr model shows the atom as a central nucleus containing protons and neutrons, with the electrons in circular orbitals at specific distances from the nucleus. These orbits form electron shells or energy levels, which are a way of visualizing the number of electrons in the outermost shells.
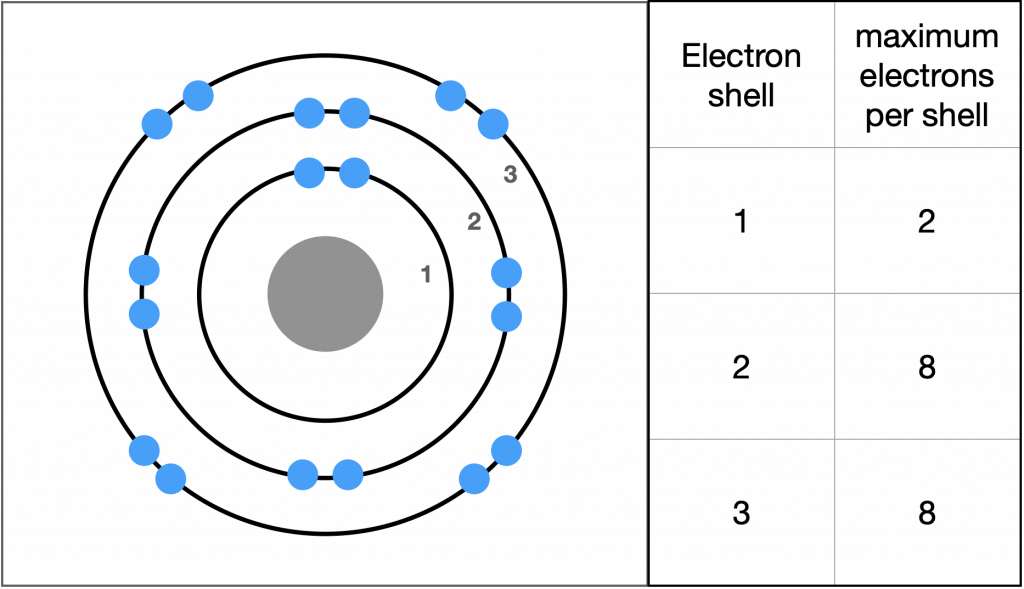
Electrons fill orbitals in a consistent order: they first fill the orbitals closest to the nucleus, then they continue to fill orbitals of increasing energy further from the nucleus. The electrons of the outermost energy level determine the atom’s energetic stability and its tendency to form chemical bonds with other atoms to form molecules.
Therefore the atoms of each particular element will have a characteristic number of valence electrons that determines the number of bonds that that particular type of atom will form. The innermost shell has a maximum of two electrons but the next two electron shells can each have a maximum of eight electrons. The outermost shell is referred to as the valence shell.
Although useful to explain the reactivity and chemical bonding of certain elements, the Bohr model does not accurately reflect how electrons spatially distribute themselves around the nucleus. They do not circle the nucleus like the earth orbits the sun; however, the Bohr model is useful in determining whether an atom will react with other atoms.
fundamental unit of matter
positively charged particle that resides in the atom's nucleus; has a mass of 1 amu and a charge of +1
uncharged particle that resides in an atom's nucleus; has a mass of 1 amu
negatively charged subatomic particle that is located outside of the nucleus in an electron orbital/shell; mass is negligible and has a negative charge of –1
electron in the outermost, or valence shell, of an atom

