23.1 Overview of Cellular Respiration
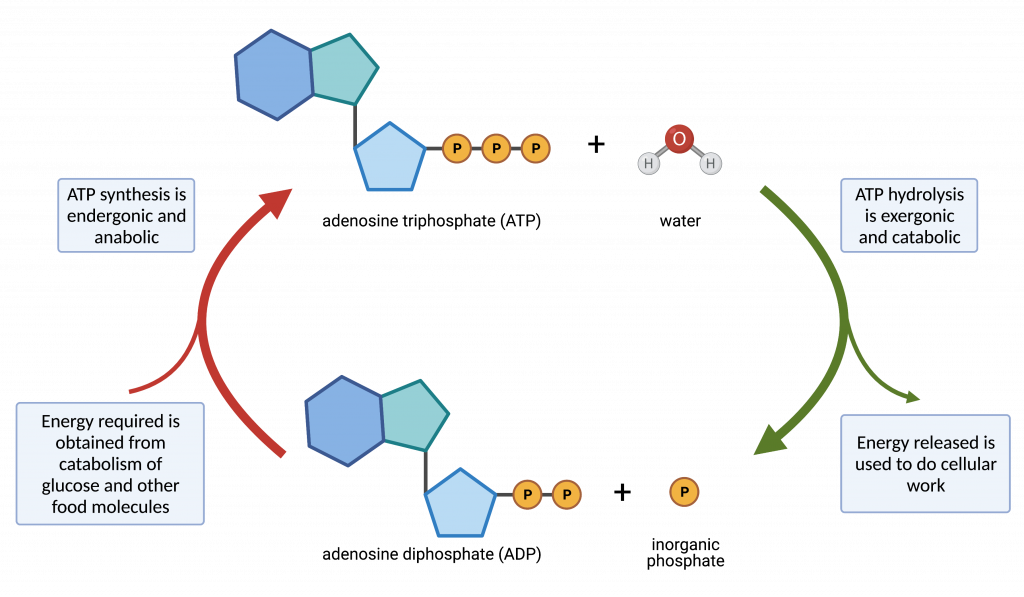
Cellular respiration is the process by which organisms break down glucose and other food molecules in a controlled, stepwise fashion for the purpose of producing ATP. Cellular respiration is exergonic; it releases energy from the chemical bonds of glucose. This exergonic process is coupled to the endergonic process of regeneration ATP from ADP and inorganic phosphate.
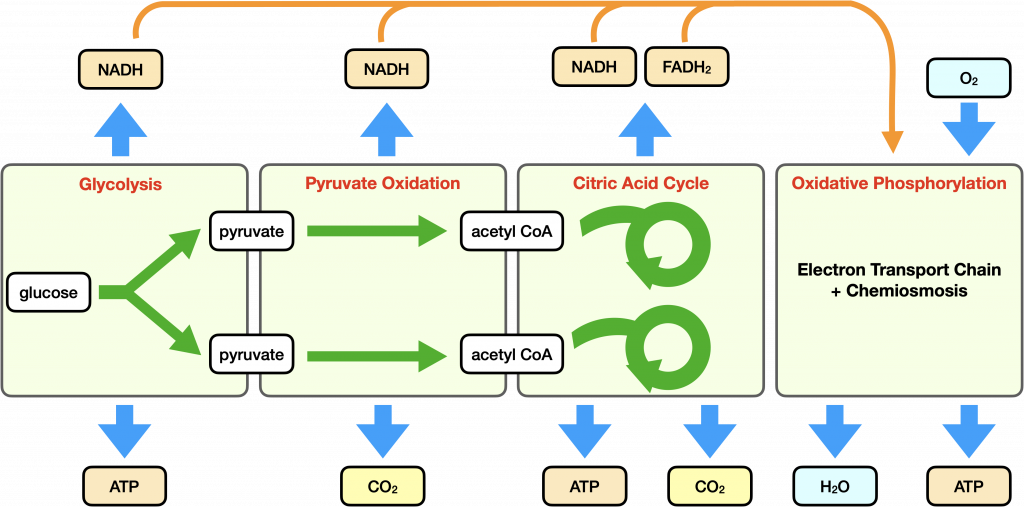
Aerobic respiration is a type of cellular respiration that requires molecular oxygen (O2). We can divide aerobic respiration into four main steps: glycolysis, pyruvate oxidation, the citric acid cycle, and oxidative phosphorylation, which includes the electron transport chain and chemiosmosis.
The first three steps of aerobic respiration harvest high energy electrons from organic molecules that can be used to power the electron transport chain. This electron transport chain maintains the hydrogen ion gradient across the mitochondrion that powers ATP synthase. High energy electrons are shuttled to the protein complexes of the electron transport chain by the electron carriers NADH and FADH2.
Electron Carriers
Electron carriers can be easily reduced (that is, they accept electrons) or oxidized (they release electrons). Nicotinamide adenine dinucleotide (NAD) is derived from vitamin B3, niacin. NAD+ is the oxidized form of the molecule; NADH is the reduced form of the molecule after it has accepted two electrons and a proton (which together are the equivalent of a hydrogen atom with an extra electron). Note that if a compound has an extra “H” on it, it is more reduced (e.g., NADH is the reduced form of NAD). A second variation of NAD, NADP, contains an extra phosphate group. Both NAD+ and FAD are extensively used in energy extraction from sugars, and NADP plays an important role in the anabolic reactions of photosynthesis in plants.

Similarly, flavin adenine dinucleotide (FAD) is derived from vitamin B2, also called riboflavin. Its reduced form is FADH2.
NAD+ + 2e– + H+ → NADH
FAD + 2e– + 2H+ → FADH2
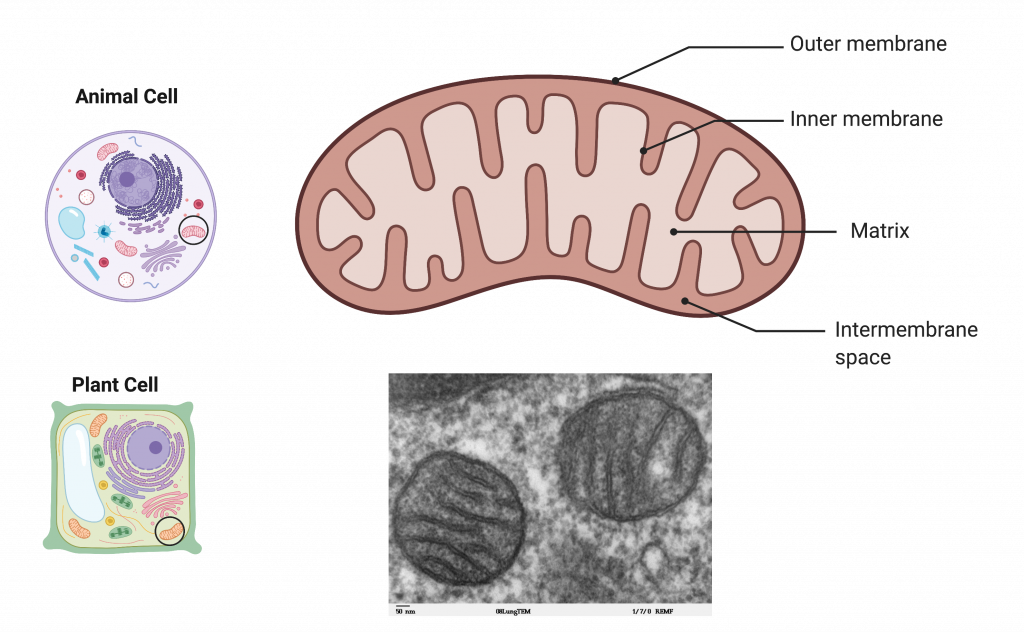
The Mitochondrion
Mitochondria are double membrane organelles that have their own ribosomes and DNA, which are derived from the evolutionary origin of mitochondria — free-living bacteria. Each membrane is a phospholipid bilayer embedded with proteins. The inner layer has folds, which increases the surface area and allows for many copies of the electron transport chain. We call the area enclosed by the inner membrane the mitochondrial matrix. The space between the inner membrane and the outer membrane is called the intermembrane space.
metabolic pathway that breaks down glucose and other food molecules to produce ATP
process in which organisms convert energy in the presence of oxygen
a series of proteins and other molecules that transfer electrons from electron donors to electron acceptors through a series of redox reactions, coupling the energy released with active transport of hydrogen ions (H+)
movement of ions down their gradient through a membrane protein; usually refers to the process that produces ATP by harnessing the energy of protons flowing down their gradient which powers ATP synthase
a molecule capable of accepting one or two electrons from one molecule and donating them to another molecule
(singular = mitochondrion) cellular organelles responsible for carrying out cellular respiration. Originally derived from proteobacteria via endosymbiosis.
the fluid-filled space inside the inner membrane of the mitochondrion
the fluid-filled space between the outer membrane and the inner membrane of the mitochondrion

