8.2 Enzyme Function
What is an Enzyme?
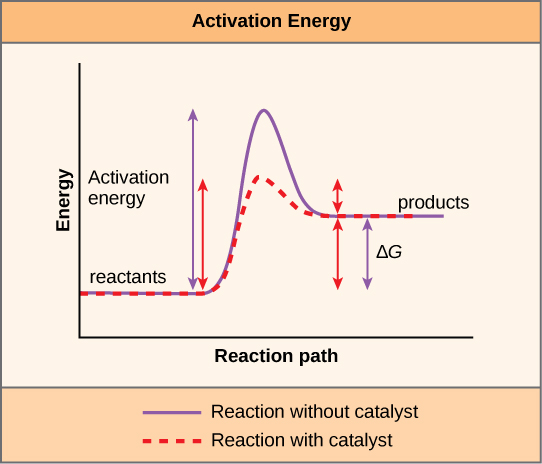
A substance that helps a chemical reaction to occur is a catalyst, and the special molecules that catalyze biochemical reactions are enzymes. Almost all enzymes are proteins and they perform the critical task of lowering the activation energies of chemical reactions inside the cell. Enzymes do this by binding to the reactant molecules, and holding them in such a way as to make the chemical bond-breaking and bond-forming processes take place more readily. It is important to remember that enzymes do not change whether a reaction is exergonic or endergonic. This is because they do not change the free energies of the reactants or products. They only reduce the activation energy required to reach the transition state. It is also important to remember that each particular enzyme functions to catalyze one particular chemical reaction.
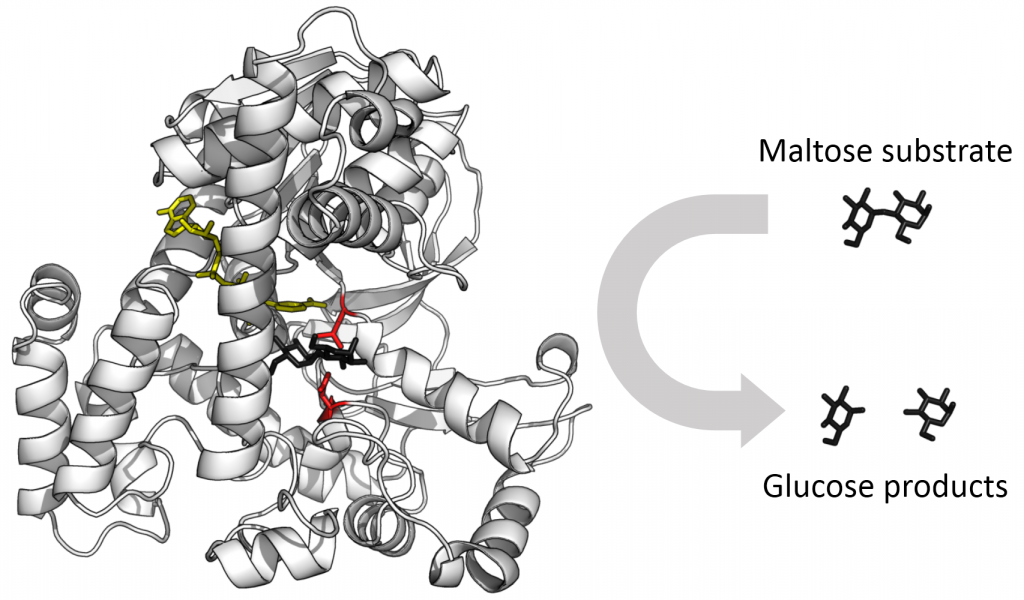
Enzyme Active Site and Substrate Specificity
The reactants to which an enzyme binds are the enzyme’s substrates. There may be one or more substrates, depending on the particular chemical reaction. In some reactions, one substrate breaks down into multiple products. In others, two substrates may come together to create one larger molecule. Two reactants might also enter a reaction, both become modified, and leave the reaction as two products. The location within the enzyme where the substrate binds is the enzyme’s active site. This is where the “action” happens. Since enzymes are proteins, there is a unique combination of amino acid side chains within the active site. Different properties characterize each residue. These can be large or small, weakly acidic or basic, hydrophilic or hydrophobic, positively or negatively charged, or neutral. The unique combination of amino acid residues, their positions, sequences, structures, and properties, creates a very specific chemical environment within the active site. This specific environment is suited to briefly bind to a specific chemical substrate (or substrates). Due to this match between an enzyme and its substrates, enzymes are known for their specificity. The “best fit” results from the shape and the amino acid functional group’s attraction to the substrate.
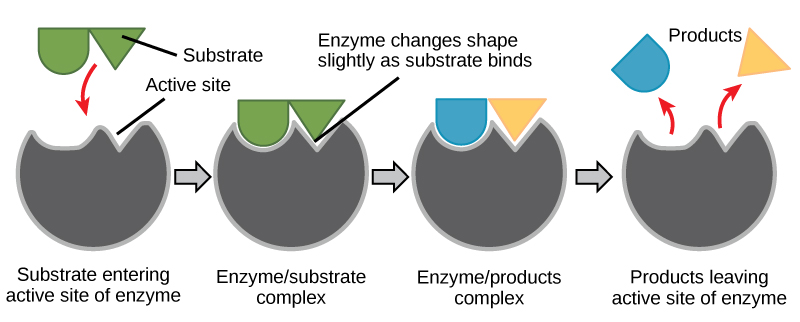
Induced Fit and Enzyme Function
For many years, scientists thought that enzyme-substrate binding took place in a simple “lock-and-key” fashion. This model asserted that the enzyme and substrate fit together perfectly in one instantaneous step. However, current research supports a more refined view scientists call induced fit. As the enzyme and substrate come together, their interaction causes a mild shift in the enzyme’s structure that confirms an ideal binding arrangement between the enzyme and the substrate’s transition state. This ideal binding maximizes the enzyme’s ability to catalyze its reaction.
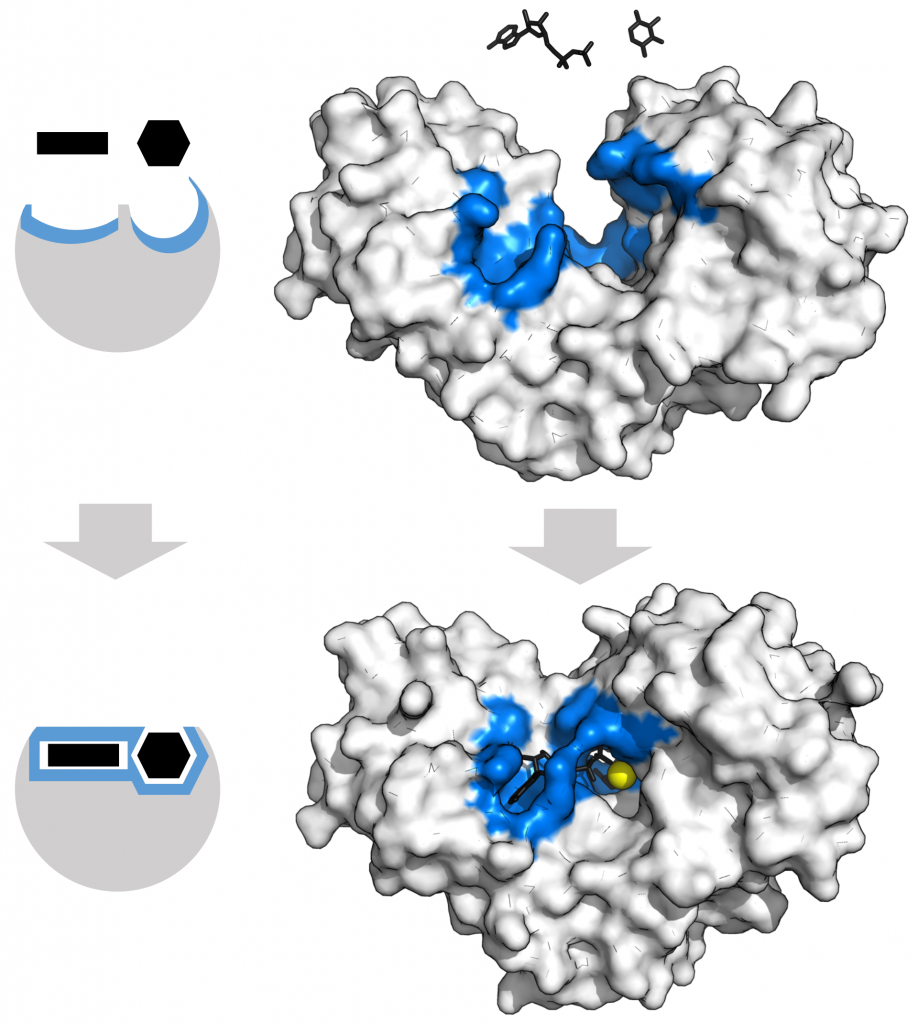
When an enzyme binds its substrate, it forms an enzyme-substrate complex. This complex lowers the reaction’s activation energy and promotes its rapid progression in one of many ways. On a basic level, enzymes promote chemical reactions that involve more than one substrate by bringing the substrates together in an optimal orientation. The appropriate region (atoms and bonds) of one molecule is close to the other molecule’s region with which it must react. Another way in which enzymes promote substrate reaction is by creating an optimal environment within the active site for the reaction to occur. Certain chemical reactions might proceed best in a slightly acidic or non-polar environment. The chemical properties that emerge from the particular arrangement of amino acid residues within an active site create the perfect environment for an enzyme’s specific substrates to react.
The enzyme-substrate complex can lower the activation energy by contorting substrate molecules in such a way as to facilitate bond-breaking, helping to reach the transition state. Finally, enzymes can also lower activation energies by taking part in the chemical reaction itself. The amino acid residues can provide certain ions or chemical groups that actually form covalent bonds with substrate molecules as a necessary step of the reaction process. When the reaction is complete, the enzyme will always return to its original state. Enzymes remain ultimately unchanged by the reactions they catalyze. After an enzyme catalyzes a reaction, it releases its product(s).
a biomolecule (usually a protein) that catalyzes chemical reactions
energy necessary for a reaction to occur
substance present at the start of a chemical reaction, and is changed into a product during the reaction
chemical reaction that releases free energy
reaction that requires an input of free energy
molecule on which an enzyme acts
the region of an enzyme where a substrate binds
dynamic fit between the enzyme and its substrate, in which both slightly modify their shapes to allow for ideal binding

