7.2 Amino Acids
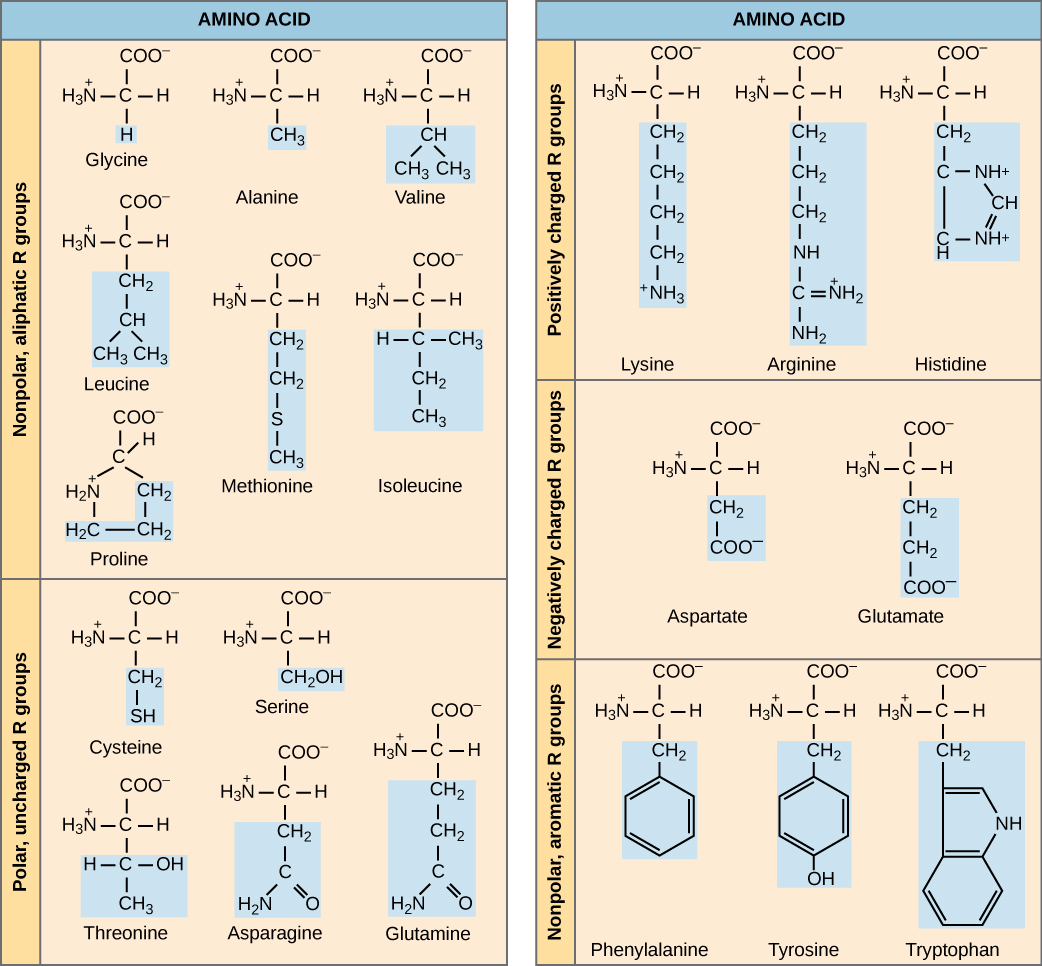
Amino acids are the monomers that comprise proteins. There are 20 common amino acids present in proteins. Each amino acid has the same fundamental structure, which consists of a central carbon atom, or the alpha (α) carbon, bonded to an amino group (NH2), a carboxyl group (COOH), and to a hydrogen atom. Every amino acid also has another atom or group of atoms bonded to the central atom, which is known as the R group.
We use the name “amino acid” because they contain both an amino group and a carboxyl group (which is acidic) in their structure. For each of the 20 amino acids, the R group (or side chain) is different.
The chemical nature of the side chain determines the amino acid’s nature (that is, whether it is acidic, basic, polar, or nonpolar). For example, the amino acid glycine has a hydrogen atom as the R group. A single upper case letter or a three-letter abbreviation is used to represent each amino acid. For example, the letter V or the three-letter symbol Val represents valine.
The sequence and the number of amino acids ultimately determine the protein’s shape, size, and function. A covalent bond, or peptide bond, attaches to each amino acid, which a dehydration reaction forms. One amino acid’s carboxyl group and the incoming amino acid’s amino group combine, releasing a water molecule. The resulting bond is the peptide bond.
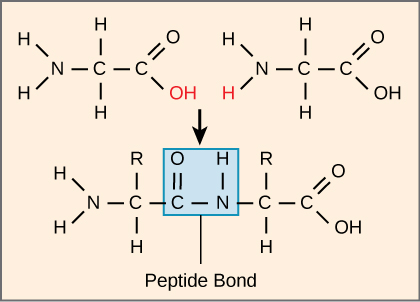
The products that such linkages form are peptides. As more amino acids join to this growing chain, the resulting chain is a polypeptide. Each polypeptide has a free amino group at one end. This end the N terminal, or the amino terminal, and the other end has a free carboxyl group, also the C or carboxyl terminal. While the terms polypeptide and protein are sometimes used interchangeably, a polypeptide is technically a polymer of amino acids, whereas the term protein is used for a polypeptide or polypeptides that have combined together, often have bound non-peptide prosthetic groups, have a distinct shape, and have a unique function.
Nine of these are essential amino acids in humans because we need them to build proteins, but the human body cannot produce them. We therefore must obtain them from our diet. Which amino acids are essential varies from organism to organism.
a protein's monomer; has a central carbon which is attached to an amino group, a carboxyl group, a hydrogen, and an R group or side chain; the R group is different for all 20 common amino acids
biological macromolecule made of amino acids; essential for cellular function
a functional group that consists of a nitrogen atom covalently bound to two hydrogen atoms (-NH2)
a functional group that consists of a carbon atom with a double covalent bond to an oxygen atom and a single covalent bond to an oxygen atom that has a single covalent bond to a hydrogen atom (-COOH)
strong bond formed between two atoms of the same or different elements; forms when electrons are shared between atoms
covalent bond formed between two amino acids by a dehydration reaction
reaction that joins monomers (or other molecules) by covalent bonds, releasing a molecule of water in the process
long chain of amino acids linked by peptide (covalent) bonds

