6.2 Functional Groups
Functional Groups
Functional groups are groups of atoms that occur within molecules and have their own specific properties. We find them attached to the “carbon backbone” of macromolecules. Chains and/or rings of carbon atoms with the occasional substitution of an element such as nitrogen or oxygen form this backbone.
The functional groups in a macromolecule are usually attached to the carbon backbone at one or several different places along its chain and/or ring structure. Each of the four types of macromolecules—proteins, lipids, carbohydrates, and nucleic acids—has its own characteristic set of functional groups that contributes greatly to its differing chemical properties and its function in living organisms.
A functional group can participate in specific chemical reactions. The figure below shows some of the important functional groups in biological molecules. They include: hydroxyl, methyl, carbonyl, carboxyl, amino, phosphate, and sulfhydryl. These groups play an important role in forming molecules like DNA, proteins, carbohydrates, and lipids.
We usually classify functional groups as hydrophobic or hydrophilic depending on their charge or polarity characteristics. An example of a hydrophobic group is the nonpolar methyl group. Among the hydrophilic functional groups is the carboxyl group that is found in amino acids, some amino acid side chains, and the fatty acids that form triglycerides and phospholipids. This carboxyl group ionizes to release hydrogen ions (H+) from the COOH group resulting in the negatively charged COO– group (still called a carboxyl group). This contributes to the hydrophilic nature of whatever molecule on which it is found. Other functional groups, such as the carbonyl group, have a partially negatively charged oxygen atom that may form hydrogen bonds with water molecules, again making the molecule more hydrophilic.
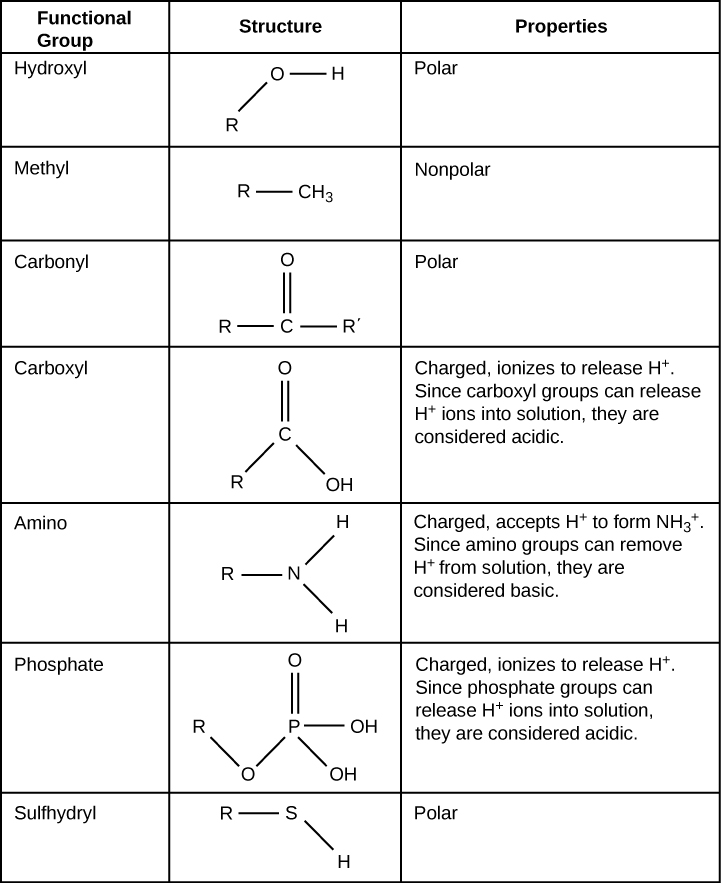
a functional group that consists of a hydrogen atom covalently bound to an oxygen atom (-OH)
a functional group that consists of a carbon atom covalently bound to three hydrogen atoms (-CH3)
a functional group that consists of a carbon atom with a double covalent bond to an oxygen atoms (-C=O)
a functional group that consists of a carbon atom with a double covalent bond to an oxygen atom and a single covalent bond to an oxygen atom that has a single covalent bond to a hydrogen atom (-COOH)
a functional group that consists of a nitrogen atom covalently bound to two hydrogen atoms (-NH2)
a functional group that consists of a phosphorus atom covalently bound to four oxygen atoms, three with a single covalent bond and one with a double covalent bond
a functional group that consists of a sulfur atom covalently bound to a hydrogen atom (-SH)

