5.2 Water’s Interactions with Other Molecules
Water’s Solvent Properties
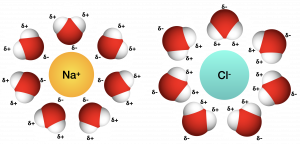
Since water is a polar molecule with slightly positive and slightly negative charges, ions and polar molecules can readily dissolve in it. Therefore, we refer to water as a solvent, a substance capable of dissolving other polar molecules and ionic compounds.
When we add ionic compounds to water, the individual ions react with the water molecules’ polar regions and their ionic bonds are disrupted in the process of dissociation. Dissociation occurs when atoms or groups of atoms break off from molecules and form ions. Consider table salt (NaCl, or sodium chloride): when we add NaCl crystals to water, the NaCl molecules dissociate into Na+ and Cl– ions,. The partially negative charge of the water molecule’s oxygen surrounds the positively charged sodium ion. The hydrogen’s partially positive charge on the water molecule surrounds the negatively charged chloride ion.
Water’s Cohesive and Adhesive Properties
Have you ever filled a glass of water to the very top and then slowly added a few more drops? Before it overflows, the water forms a dome-like shape above the rim of the glass. This water can stay above the glass because of the property of cohesion. In cohesion, water molecules are attracted to each other (because of hydrogen bonding), keeping the molecules together at the liquid-gas (water-air) interface, although there is no more room in the glass.
Cohesion allows for surface tension, the capacity of a substance to withstand rupturing when placed under tension or stress. This is also why water forms droplets when on a dry surface rather than flattening by gravity. Insects such as the water strider use the water’s surface tension to stay afloat on the water’s surface layer and even mate there.

Water is also attracted to other charged and polar molecules. We call this property adhesion. This attraction is sometimes stronger than water’s cohesive forces, especially when the water is exposed to charged surfaces such as those on the inside of thin glass tubes known as capillary tubes. We observe adhesion when water “climbs” up the tube placed in a glass of water: notice that the water appears to be higher on the tube’s sides than in the middle. This is because the water molecules are attracted to the capillary’s charged glass walls more than they are to each other and therefore adhere to it.
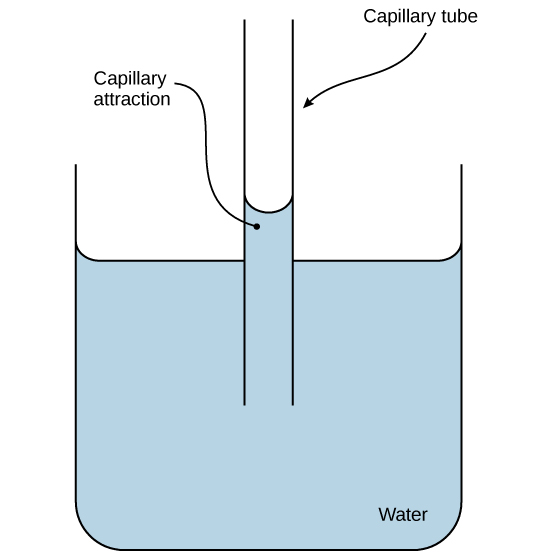
Adhesive forces are important for transporting water from the roots to the leaves in plants. These forces create a “pull” on the water column. This pull results from the tendency of water molecules evaporating on the plant’s surface to stay connected to water molecules below them, and so they are pulled along. Plants use this natural phenomenon to help transport water from their roots to their leaves. Without these properties of water, plants would be unable to receive the water and the dissolved minerals they require.
substance capable of dissolving another substance
attraction between molecules of the same type
attraction between molecules of different types; for example, attraction of water and other polar molecules

