5.1 Water is Polar
Water is one of the more abundant molecules on our planet, and the one most critical to life. Water comprises approximately 60–70 percent of the human body. Without it, life as we know it simply would not exist.
One of water’s important properties is that it is composed of polar molecules: the hydrogen and oxygen within water molecules (H2O) form polar covalent bonds. While there is no net charge to a water molecule overall, if we look more closely, there is a slightly positive charge on hydrogen and a slightly negative charge on oxygen. Water generates charges because oxygen is more electronegative than hydrogen, making it more likely that a shared electron would be near the oxygen nucleus than the hydrogen nucleus, thus generating the partial negative charge near the oxygen.
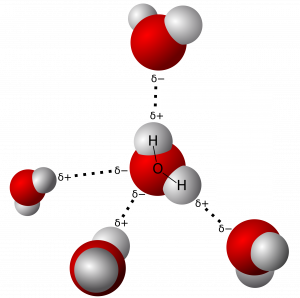
As a result of water’s polarity, each water molecule attracts other water molecules because of the opposite charges between water molecules, forming hydrogen bonds. Water also attracts or is attracted to other polar molecules and ions. We call a polar substance that interacts readily with or dissolves in water hydrophilic (hydro- = “water”; -philic = “loving”). In contrast, nonpolar molecules such as oils and fats do not interact well with water. A good example of this is vinegar and oil salad dressing (an acidic water solution). We call such nonpolar compounds hydrophobic (hydro- = “water”; -phobic = “fearing”).
molecules that interact well with water, such as ions and polar molecules
molecules that do not interact well with water; nonpolar molecules

