4.3 Covalent Bonds
Chemical Bonds
All elements are most stable when their outermost shell is filled with electrons. This is because it is energetically favorable for atoms to be in that configuration and it makes them stable. However, since not all elements have enough electrons to fill their outermost shells, atoms form chemical bonds with other atoms thereby obtaining the electrons they need to attain a stable electron configuration. When two or more atoms chemically bond with each other, the resultant chemical structure is a molecule or compound. Atoms can form molecules by donating, accepting, or sharing electrons to fill their outer shells.
Covalent Bonds
Atoms can share electrons to form covalent bonds. These bonds are stronger and much more common than ionic bonds in the molecules of living organisms. We commonly find covalent bonds in carbon-based organic molecules, such as our DNA and proteins. We also find covalent bonds in inorganic molecules like H2O, CO2, and O2. The bonds may share one, two, or three pairs of electrons, making single, double, and triple bonds, respectively. The more covalent bonds between two atoms, the stronger their connection. Thus, triple bonds are the strongest.
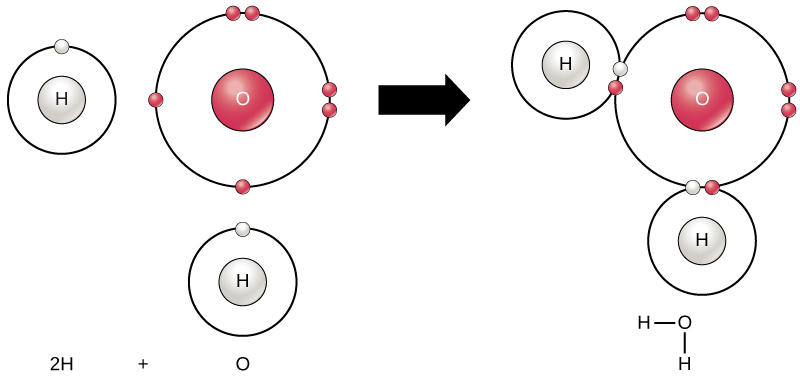
Forming water molecules provides an example of covalent bonding. Covalent bonds bind the hydrogen and oxygen atoms that combine to form water molecules. The electron from the hydrogen splits its time between the hydrogen atoms’ incomplete outer shell and the oxygen atoms’ incomplete outer shell. To completely fill the oxygen’s outer shell, which has six electrons but which would be more stable with eight, two electrons (one from each hydrogen atom) are needed: hence, the well-known formula H2O. The two elements share the electrons to fill the outer shell of each, making both elements more stable.
Nonpolar Covalent Bonds
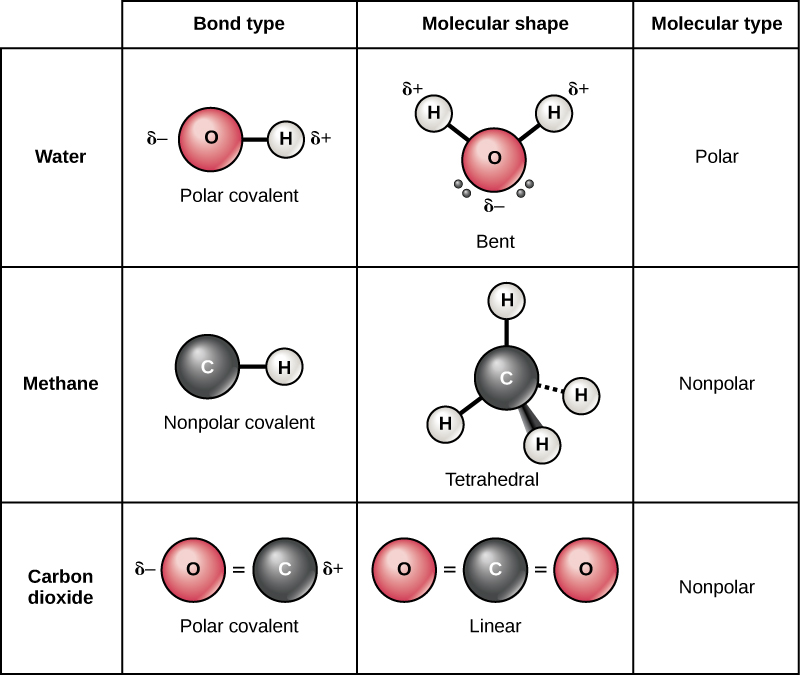
There are two types of covalent bonds: polar and nonpolar. Nonpolar covalent bonds form between two atoms of the same element or between different elements that share electrons equally. For example, molecular oxygen (O2) is nonpolar because the electrons distribute equally between the two oxygen atoms.
Methane (CH4) is another example of a molecule formed by nonpolar covalent bonds. Carbon has four electrons in its outermost shell and needs four more to fill it. It obtains these four from four hydrogen atoms, each atom providing one, making a stable outer shell of eight electrons. Carbon and hydrogen do not have the same electronegativity but are similar; thus, nonpolar bonds form. The hydrogen atoms each need one electron for their outermost shell, which is filled when it contains two electrons. These elements share the electrons equally among the carbons and the hydrogen atoms, creating a nonpolar molecule.
Polar Covalent Bonds
In a polar covalent bond, atoms unequally share the electrons, meaning that the shared electrons are attracted more to one nucleus than the other. Because of the unequal electron distribution between the atoms of different elements, one of the atoms will develop a slightly positive (δ+) charge and the other a slightly negative (δ–) charge. This partial charge is an important property of water and accounts for many of its characteristics.
Water is a polar molecule, with the hydrogen atoms acquiring a partial positive charge and the oxygen a partial negative charge. This occurs because the oxygen atom’s nucleus is more attractive to the hydrogen atoms’ electrons than the hydrogen nucleus is to the oxygen’s electrons. Thus, oxygen has a higher electronegativity than hydrogen and the shared electrons spend more time near the oxygen nucleus than the hydrogen atoms’ nucleus, giving the oxygen and hydrogen atoms slightly negative and positive charges, respectively. Partial charges develop when one element is significantly more electronegative than the other, and the charges that these polar bonds generate may then be used to form hydrogen bonds based on the attraction of opposite partial charges. Since macromolecules often have atoms within them that differ in electronegativity, polar bonds are often present in organic molecules.
interaction between two or more of the same or different atoms that results in forming molecules
chemical structure consisting of two or more atoms held together by chemical bonds
substance composed of molecules consisting of atoms of at least two different elements
strong bond formed between two atoms of the same or different elements; forms when electrons are shared between atoms
covalent bond in which one pair of electrons is shared
covalent bond in which two pairs of electrons are shared
covalent bond in which three pairs of electrons are shared
type of covalent bond that forms between atoms when electrons are shared equally between them
ability of an atom to attract electrons
type of covalent bond that forms as a result of unequal electron sharing, resulting in creating slightly positive and negative charged molecule regions

