4.1 The Composition of Organisms
At its most fundamental level, life is made up of matter. Matter is any substance that occupies space and has mass. Elements are unique forms of matter with specific chemical and physical properties that cannot break down into smaller substances by ordinary chemical reactions. There are 118 elements, but only 98 occur naturally. The remaining elements are unstable and require scientists to synthesize them in laboratories.
Each element is designated by its chemical symbol, which is a single capital letter or, when the first letter is already “taken” by another element, a combination of two letters. Some elements follow the English term for the element, such as C for carbon and Ca for calcium. Other elements’ chemical symbols derive from their Latin names. For example, the symbol for sodium is Na, referring to natrium, the Latin word for sodium.
The four elements that are most abundant in living organisms are oxygen (O), carbon (C), hydrogen (H), and nitrogen (N). Phosphorus (P) and sulfur (S) are also extremely important to biomolecules.
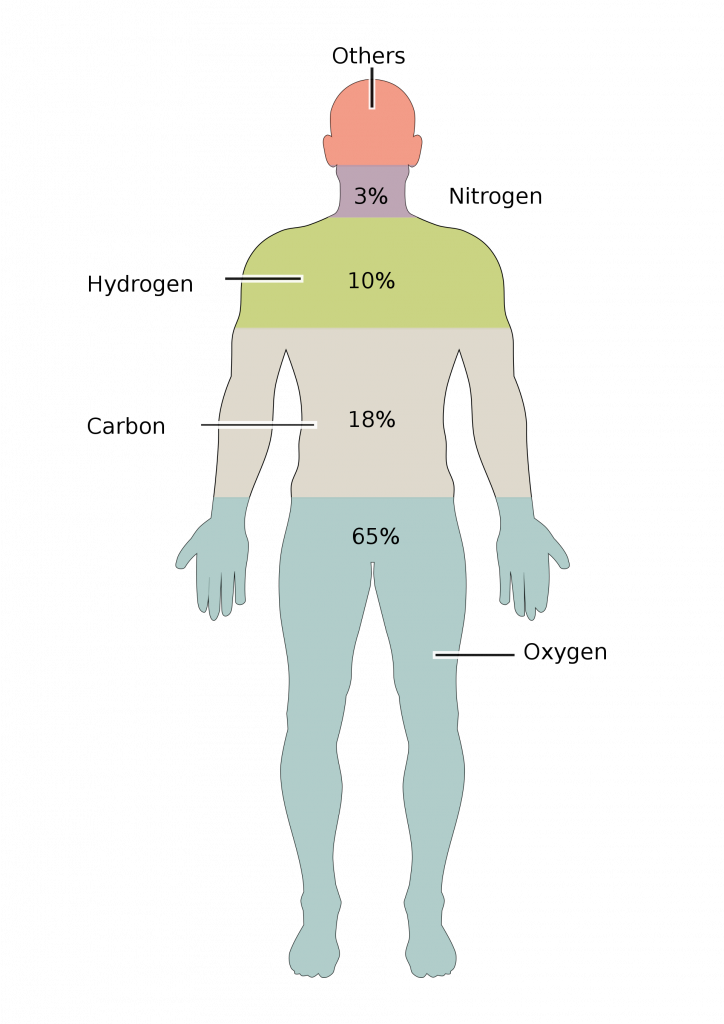
The reason these atoms are so abundant in humans (and organisms in general) is because they compose the molecules that make up living organisms. The most abundant molecule in the body is water, which comprises about 65% of the weight of a human. Most of the remainder is made up of biological macromolecules. The most abundant and important of these are proteins, nucleic acids, lipids, and carbohydrates.
form of matter with specific chemical and physical properties that cannot break down into smaller substances by ordinary chemical reactions
biological macromolecule made of amino acids; essential for cellular function
biological macromolecule that functions in information storage and transfer; DNA or RNA
macromolecule that is nonpolar and hydrophobic
biological macromolecule in which the ratio of carbon to hydrogen and to oxygen is 1:2:1; serve as energy sources and structural support molecules

