6.1 Hydrocarbons
Carbon
Cells are made of complex molecules called macromolecules. The most important of these are: proteins, nucleic acids (RNA and DNA), carbohydrates, and lipids. The macromolecules are a subset of organic molecules (any carbon-containing liquid, solid, or gas) that are especially important for life. The fundamental component for all of these macromolecules is carbon. The carbon atom can form covalent bonds to as many as four different atoms, making it ideal to serve as the basic structural component, or “backbone,” of the macromolecules.
Individual carbon atoms have an incomplete outermost electron shell. With an atomic number of 6 (six electrons and six protons), the first two electrons fill the inner shell, leaving four in the second shell. Therefore, carbon atoms can form up to four covalent bonds with other atoms. The methane molecule provides an example: it has the chemical formula CH4. Each of its four hydrogen atoms forms a single covalent bond with the carbon atom by sharing a pair of electrons. This results in a filled outermost shell.
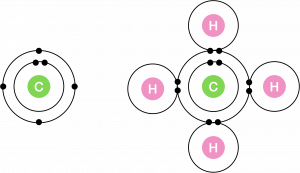
Hydrocarbons
Hydrocarbons are organic molecules consisting entirely of carbon and hydrogen, such as methane (CH4) described above. We often use hydrocarbons in our daily lives as fuels—like the propane in a gas grill or the butane in a lighter. The many covalent bonds between the atoms in hydrocarbons store a great amount of energy, which releases when these molecules burn (oxidize). For example, methane, the simplest hydrocarbon molecule, is an excellent fuel.
As the backbone of the large molecules of living things, hydrocarbons may exist as linear carbon chains, branched carbon chains, carbon rings, or combinations of these. Furthermore, individual carbon-to-carbon bonds may be single, double, or triple covalent bonds, and each type of bond affects the molecule’s geometry in a specific way. The three-dimensional shape of biological macromolecules is critical to how they function.
Hydrocarbon Chains
Bonds between carbon atoms form hydrocarbon chains. Hydrocarbon chains may be branched or unbranched. The arrangement of single, double, and triple covalent bonds within a molecule determines the overall geometry of the molecule.
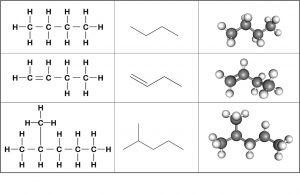
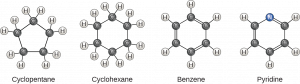
Hydrocarbon Rings
Another type of hydrocarbon consists of closed rings of carbon atoms. The carbon atoms can form single or double bonds with other carbons. Examples of biological molecules that incorporate ring structures include some amino acids and the hormones estrogen and testosterone. Carbon can form ring structures, such as those seen here. Carbon atoms within these rings may interact with one another by single or double covalent bonds. Other atoms may also be incorporated into the ring, as shown by pyridine (right).
biological macromolecule made of amino acids; essential for cellular function
biological macromolecule that functions in information storage and transfer; DNA or RNA
biological macromolecule in which the ratio of carbon to hydrogen and to oxygen is 1:2:1; serve as energy sources and structural support molecules
macromolecule that is nonpolar and hydrophobic
an organic molecule consisting of carbon and hydrogen

