6 Chapter 6: Cellular Communication

Figure 6.1 Have you ever become separated from a friend while in a crowd? If so, you know the challenge of searching for someone when surrounded by thousands of other people. If you and your friend have cell phones, your chances of finding each other are good. A cell phone’s ability to send and receive messages makes it an ideal communication device. (credit: modification of work by Vincent and Bella Productions)
Introduction
Imagine what life would be like if you and the people around you could not communicate. You would not be able to express your wishes to others, nor could you ask questions to find out more about your environment. Social organization is dependent on communication between the individuals that comprise that society; without communication, society would fall apart.
While the necessity for cellular communication in larger organisms seems obvious, even single-celled organisms communicate with each other. Yeast cells signal each other to aid mating. Some forms of bacteria coordinate their actions in order to form large complexes called biofilms or to organize the production of toxins to remove competing organisms. The ability of cells to communicate through chemical signals originated in single cells and was essential for the evolution of multicellular organisms. The efficient and error-free function of communication systems is vital for all life as we know it.
Learning Objectives
You will be able to describe these characteristics of organisms:
- Composed of biomolecules
- Metabolize
- Composed of cells
- Maintain homeostasis
- Respond to external stimuli
- Grow and reproduce
- Evolve
6.1 | Signaling Molecules and Cellular Receptors
There are two kinds of communication in the world of living cells. Communication between cells is called intercellular signaling, and communication within a cell is called intracellular signaling. An easy way to remember the distinction is by understanding the Latin origin of the prefixes: inter- means “between” (for example, intersecting lines are those that cross each other) and intra- means “inside” (like intravenous).
Chemical signals are released by signaling cells in the form of small, usually volatile or soluble molecules called ligands. A ligand is a molecule that binds another specific molecule, in some cases, delivering a signal in the process. Ligands can thus be thought of as signaling molecules. Ligands interact with proteins in target cells, which are cells that are affected by chemical signals; these proteins are also called receptors. Ligands and receptors exist in several varieties; however, a specific ligand will have a specific receptor that typically binds only that ligand.
Forms of Signaling
There are four categories of chemical signaling found in multicellular organisms: paracrine signaling, endocrine signaling, autocrine signaling, and direct signaling across gap junctions (Figure 9.2). The main difference between the different categories of signaling is the distance that the signal travels through the organism to reach the target cell. Not all cells are affected by the same signals.
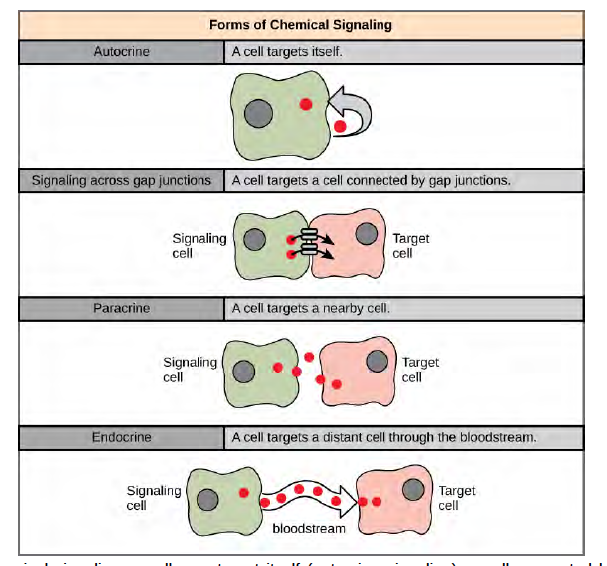
Figure 6.2 In chemical signaling, a cell may target itself (autocrine signaling), a cell connected by gap junctions, a nearby cell (paracrine signaling), or a distant cell (endocrine signaling). Paracrine signaling acts on nearby cells, endocrine signaling uses the circulatory system to transport ligands, and autocrine signaling acts on the signaling cell. Signaling via gap junctions involves signaling molecules moving directly between adjacent cells.
Paracrine Signaling
Signals that act locally between cells that are close together are called paracrine signals. Paracrine signals move by diffusion through the extracellular matrix. These types of signals usually elicit quick responses that last only a short amount of time. In order to keep the response localized, paracrine ligand molecules are normally quickly degraded by enzymes or removed by neighboring cells. Removing the signals will reestablish the concentration gradient for the signal, allowing them to quickly diffuse through the intracellular space if released again.
When the neurotransmitter binds the receptor on the surface of the postsynaptic cell, the electrochemical potential of the target cell changes, and the next electrical impulse is launched. The neurotransmitters that are released into the chemical synapse are degraded quickly or get reabsorbed by the presynaptic cell so that the recipient nerve cell can recover quickly and be prepared to respond rapidly to the next synaptic signal.
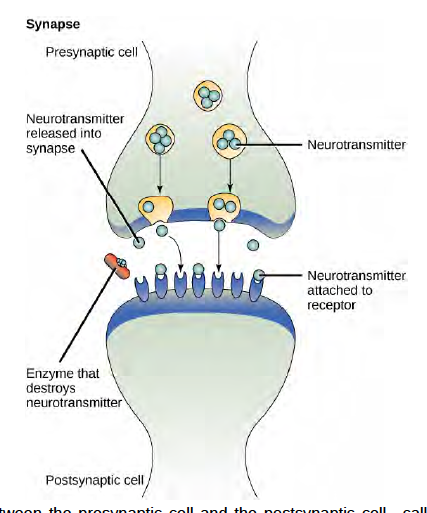
Figure 6.3 The distance between the presynaptic cell and the postsynaptic cell—called the synaptic gap—is very small and allows for rapid diffusion of the neurotransmitter. Enzymes in the synapatic cleft degrade some types of neurotransmitters to terminate the signal.
Endocrine Signaling
Signals from distant cells are called endocrine signals, and they originate from endocrine cells. (In the body, many endocrine cells are located in endocrine glands, such as the thyroid gland, the hypothalamus, and the pituitary gland.) These types of signals usually produce a slower response but have a longer-lasting effect. The ligands released in endocrine signaling are called hormones, signaling molecules that are produced in one part of the body but affect other body regions some distance away.
Autocrine Signaling
Autocrine signals are produced by signaling cells that can also bind to the ligand that is released. This means the signaling cell and the target cell can be the same or a similar cell (the prefix auto- means self, a reminder that the signaling cell sends a signal to itself). This type of signaling often occurs during the early development of an organism to ensure that cells develop into the correct tissues and take on the proper function. Autocrine signaling also regulates pain sensation and inflammatory responses. Further, if a cell is infected with a virus, the cell can signal itself to undergo programmed cell death, killing the virus in the process. In some cases, neighboring cells of the same type are also influenced by the released ligand. In embryological development, this process of stimulating a group of neighboring cells may help to direct the differentiation of identical cells into the same cell type, thus ensuring the proper developmental outcome.
Direct Signaling Across Gap Junctions
Gap junctions in animals and plasmodesmata in plants are connections between the plasma membranes of neighboring cells. These water-filled channels allow small signaling molecules, called intracellular mediators, to diffuse between the two cells. Small molecules, such as calcium ions (Ca2+), are able to move between cells, but large molecules like proteins and DNA cannot fit through the channels. The specificity of the channels ensures that the cells remain independent but can quickly and easily transmit signals. The transfer of signaling molecules communicates the current state of the cell that is directly next to the target cell; this allows a group of cells to coordinate their response to a signal that only one of them may have received. In plants, plasmodesmata are ubiquitous, making the entire plant into a giant, communication network.
Types of Receptors
Receptors are protein molecules in the target cell or on its surface that bind ligand. There are two types of receptors, internal receptors and cell-surface receptors.
Internal receptors
Internal receptors, also known as intracellular or cytoplasmic receptors, are found in the cytoplasm of the cell and respond to hydrophobic ligand molecules that are able to travel across the plasma membrane. Once inside the cell, many of these molecules bind to proteins that act as regulators of mRNA synthesis (transcription) to mediate gene expression. Gene expression is the cellular process of transforming the information in a cell’s DNA into a sequence of amino acids, which ultimately forms a protein. When the ligand binds to the internal receptor, a conformational change is triggered that exposes a DNA-binding site on the protein. The ligand-receptor complex moves into the nucleus, then binds to specific regulatory regions of the chromosomal DNA and promotes the initiation of transcription (Figure 9.4). Transcription is the process of copying the information in a cells DNA into a special form of RNA called messenger RNA (mRNA); the cell uses information in the mRNA (which moves out into the cytoplasm and associates with ribosomes) to link specific amino acids in the correct order, producing a protein. Internal receptors can directly influence gene expression without having to pass the signal on to other receptors or messengers.
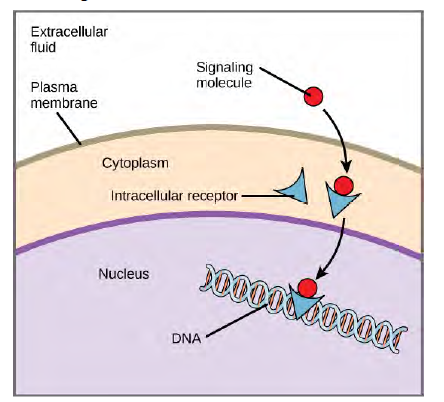
Figure 6.4 Hydrophobic signaling molecules typically diffuse across the plasma membrane and interact with intracellular receptors in the cytoplasm. Many intracellular receptors are transcription factors that interact with DNA in the nucleus and regulate gene expression.
Cell-Surface Receptors
Cell-surface receptors, also known as transmembrane receptors, are cell surface, membrane-anchored (integral) proteins that bind to external ligand molecules. This type of receptor spans the plasma membrane and performs signal transduction, in which an extracellular signal is converted into an intercellular signal. Ligands that interact with cell-surface receptors do not have to enter the cell that they affect. Cell-surface receptors are also called cell-specific proteins or markers because they are specific to individual cell types.
Because cell-surface receptor proteins are fundamental to normal cell functioning, it should come as no surprise that a malfunction in any one of these proteins could have severe consequences. Errors in the protein structures of certain receptor molecules have been shown to play a role in hypertension (high blood pressure), asthma, heart disease, and cancer.
Each cell-surface receptor has three main components: an external ligand-binding domain, a hydrophobic membrane- spanning region, and an intracellular domain inside the cell. The ligand-binding domain is also called the extracellular domain. The size and extent of each of these domains vary widely, depending on the type of receptor.
- A. B. Sigalov, The School of Nature. IV. Learning from Viruses, Self/Nonself 1, no. 4 (2010): 282-298. Y. Cao, X. Koh, L. Dong, X. Du, A. Wu, X. Ding, H. Deng, Y. Shu, J. Chen, T. Jiang, Rapid Estimation of Binding Activity of Influenza Virus Hemagglutinin to Human and Avian Receptors, PLoS One 6, no. 4 (2011): e18664.
Cell-surface receptors are involved in most of the signaling in multicellular organisms. There are three general categories of cell-surface receptors: ion channel-linked receptors, G-protein-linked receptors, and enzyme-linked receptors.
Ion channel-linked receptors bind a ligand and open a channel through the membrane that allows specific ions to pass through. To form a channel, this type of cell-surface receptor has an extensive membrane-spanning region. In order to interact with the phospholipid fatty acid tails that form the center of the plasma membrane, many of the amino acids in the membrane-spanning region are hydrophobic in nature. Conversely, the amino acids that line the inside of the channel are hydrophilic to allow for the passage of water or ions. When a ligand binds to the extracellular region of the channel, there is a conformational change in the protein’s structure that allows ions such as sodium, calcium, magnesium, and hydrogen to pass through (Figure 9.5).
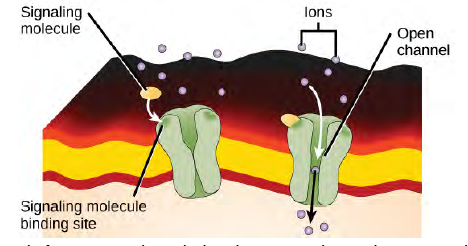
Figure 6.5 Gated ion channels form a pore through the plasma membrane that opens when the signaling molecule binds. The open pore then allows ions to flow into or out of the cell.
G-protein-linked receptors bind a ligand and activate a membrane protein called a G-protein. The activated G-protein then interacts with either an ion channel or an enzyme in the membrane (Figure 9.6). All G-protein-linked receptors have seven transmembrane domains, but each receptor has its own specific extracellular domain and G-protein-binding site.
Cell signaling using G-protein-linked receptors occurs as a cyclic series of events. Before the ligand binds, the inactive G- protein can bind to a newly revealed site on the receptor specific for its binding. Once the G-protein binds to the receptor, the resultant shape change activates the G-protein, which releases GDP and picks up GTP. The subunits of the G-protein then split into the α subunit and the βγ subunit. One or both of these G-protein fragments may be able to activate other proteins as a result. After awhile, the GTP on the active α subunit of the G-protein is hydrolyzed to GDP and the βγ subunit is deactivated. The subunits reassociate to form the inactive G-protein and the cycle begins anew.
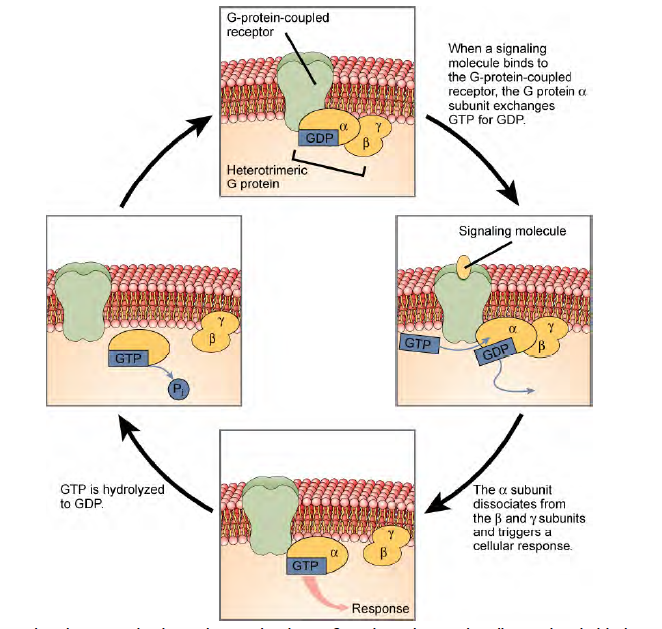
Figure 6.6 Heterotrimeric G proteins have three subunits: α, β, and γ. When a signaling molecule binds to a G-protein- coupled receptor in the plasma membrane, a GDP molecule associated with the α subunit is exchanged for GTP. The β and γ subunits dissociate from the α subunit, and a cellular response is triggered either by the α subunit or the dissociated βγ pair. Hydrolysis of GTP to GDP terminates the signal.
G-protein-linked receptors have been extensively studied and much has been learned about their roles in maintaining health. Bacteria that are pathogenic to humans can release poisons that interrupt specific G-protein-linked receptor function, leading to illnesses such as pertussis, botulism, and cholera. In cholera (Figure 9.7), for example, the water-borne bacterium Vibrio cholerae produces a toxin, choleragen, that binds to cells lining the small intestine. The toxin then enters these intestinal cells, where it modifies a G-protein that controls the opening of a chloride channel and causes it to remain continuously active, resulting in large losses of fluids from the body and potentially fatal dehydration as a result.

Figure 6.7 Transmitted primarily through contaminated drinking water, cholera is a major cause of death in the developing world and in areas where natural disasters interrupt the availability of clean water. The cholera bacterium, Vibrio cholerae, creates a toxin that modifies G-protein-mediated cell signaling pathways in the intestines. Modern sanitation eliminates the threat of cholera outbreaks, such as the one that swept through New York City in 1866. This poster from that era shows how, at that time, the way that the disease was transmitted was not understood. (credit: New York City Sanitary Commission)
Enzyme-linked receptors are cell-surface receptors with intracellular domains that are associated with an enzyme. In some cases, the intracellular domain of the receptor itself is an enzyme. Other enzyme-linked receptors have a small intracellular domain that interacts directly with an enzyme. The enzyme-linked receptors normally have large extracellular and intracellular domains, but the membrane-spanning region consists of a single alpha-helical region of the peptide strand. When a ligand binds to the extracellular domain, a signal is transferred through the membrane, activating the enzyme. Activation of the enzyme sets off a chain of events within the cell that eventually leads to a response. One example of this type of enzyme-linked receptor is the tyrosine kinase receptor (Figure 9.8). A kinase is an enzyme that transfers phosphate groups from ATP to another protein. The tyrosine kinase receptor transfers phosphate groups to tyrosine molecules (tyrosine residues). First, signaling molecules bind to the extracellular domain of two nearby tyrosine kinase receptors. The two neighboring receptors then bond together, or dimerize. Phosphates are then added to tyrosine residues on the intracellular domain of the receptors (phosphorylation). The phosphorylated residues can then transmit the signal to the next messenger within the cytoplasm.
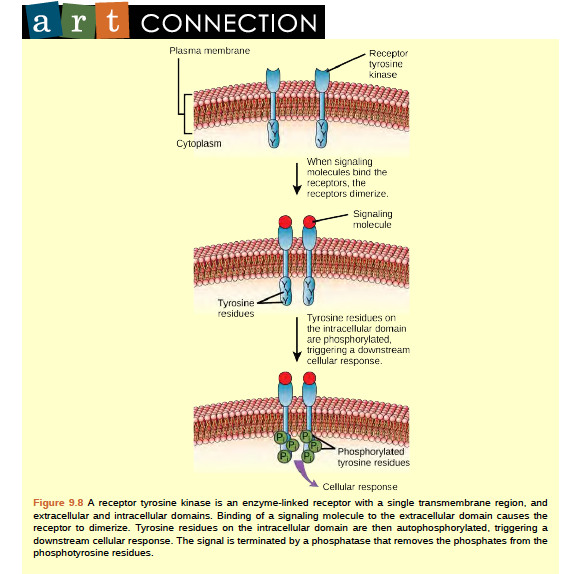
Figure 6.8 A receptor tyrosine kinase is an enzyme-linked receptor with a single transmembrane region, and extracellular and intracellular domains. Binding of a signaling molecule to the extracellular domain causes the receptor to dimerize. Tyrosine residues on the intracellular domain are then autophosphorylated, triggering a downstream cellular response. The signal is terminated by a phosphatase that removes the phosphates from the phosphotyrosine residues.
HER2 is a receptor tyrosine kinase. In 30 percent of human breast cancers, HER2 is permanently activated, resulting in unregulated cell division. Lapatinib, a drug used to treat breast cancer, inhibits HER2 receptor tyrosine kinase autophosphorylation (the process by which the receptor adds phosphates onto itself), thus reducing tumor growth by 50 percent. Besides autophosphorylation, which of the following steps would be inhibited by Lapatinib?
- Signaling molecule binding, dimerization, and the downstream cellular response
- Dimerization, and the downstream cellular response
- The downstream cellular response
- Phosphatase activity, dimerization, and the downstream cellular response
Signaling Molecules
Produced by signaling cells and the subsequent binding to receptors in target cells, ligands act as chemical signals that travel to the target cells to coordinate responses. The types of molecules that serve as ligands are incredibly varied and range from small proteins to small ions like calcium (Ca2+).
Small Hydrophobic Ligands
Small hydrophobic ligands can directly diffuse through the plasma membrane and interact with internal receptors. Important members of this class of ligands are the steroid hormones. Steroids are lipids that have a hydrocarbon skeleton with four fused rings; different steroids have different functional groups attached to the carbon skeleton. Steroid hormones include the female sex hormone, estradiol, which is a type of estrogen; the male sex hormone, testosterone; and cholesterol, which is an important structural component of biological membranes and a precursor of steriod hormones (Figure 9.9). Other hydrophobic hormones include thyroid hormones and vitamin D. In order to be soluble in blood, hydrophobic ligands must bind to carrier proteins while they are being transported through the bloodstream.
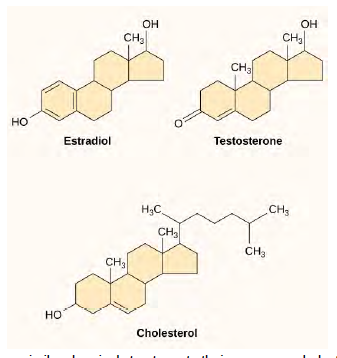
Figure 6.9 Steroid hormones have similar chemical structures to their precursor, cholesterol. Because these molecules are small and hydrophobic, they can diffuse directly across the plasma membrane into the cell, where they interact with internal receptors.
Water-Soluble Ligands
Water-soluble ligands are polar and therefore cannot pass through the plasma membrane unaided; sometimes, they are too large to pass through the membrane at all. Instead, most water-soluble ligands bind to the extracellular domain of cell- surface receptors. This group of ligands is quite diverse and includes small molecules, peptides, and proteins.
Other Ligands
Nitric oxide (NO) is a gas that also acts as a ligand. It is able to diffuse directly across the plasma membrane, and one of its roles is to interact with receptors in smooth muscle and induce relaxation of the tissue. NO has a very short half-life and therefore only functions over short distances. Nitroglycerin, a treatment for heart disease, acts by triggering the release of NO, which causes blood vessels to dilate (expand), thus restoring blood flow to the heart. NO has become better known recently because the pathway that it affects is targeted by prescription medications for erectile dysfunction, such as Viagra (erection involves dilated blood vessels).
6.2 | Propagation of the Signal
Once a ligand binds to a receptor, the signal is transmitted through the membrane and into the cytoplasm. Continuation of a signal in this manner is called signal transduction. Signal transduction only occurs with cell-surface receptors because internal receptors are able to interact directly with DNA in the nucleus to initiate protein synthesis.
When a ligand binds to its receptor, conformational changes occur that affect the receptor’s intracellular domain. Conformational changes of the extracellular domain upon ligand binding can propagate through the membrane region of the receptor and lead to activation of the intracellular domain or its associated proteins. In some cases, binding of the ligand causes dimerization of the receptor, which means that two receptors bind to each other to form a stable complex called a dimer. A dimer is a chemical compound formed when two molecules (often identical) join together. The binding of the receptors in this manner enables their intracellular domains to come into close contact and activate each other.
Binding Initiates a Signaling Pathway
After the ligand binds to the cell-surface receptor, the activation of the receptor’s intracellular components sets off a chain of events that is called a signaling pathway or a signaling cascade. In a signaling pathway, second messengers, enzymes, and activated proteins interact with specific proteins, which are in turn activated in a chain reaction that eventually leads to a change in the cell’s environment (Figure 9.10). The events in the cascade occur in a series, much like a current flows in a river. Interactions that occur before a certain point are defined as upstream events, and events after that point are called downstream events.

Signaling pathways can get very complicated very quickly because most cellular proteins can affect different downstream events, depending on the conditions within the cell. A single pathway can branch off toward different endpoints based on the interplay between two or more signaling pathways, and the same ligands are often used to initiate different signals in different cell types. This variation in response is due to differences in protein expression in different cell types. Another complicating element is signal integration of the pathways, in which signals from two or more different cell-surface receptors merge to activate the same response in the cell. This process can ensure that multiple external requirements are met before a cell commits to a specific response.
The effects of extracellular signals can also be amplified by enzymatic cascades. At the initiation of the signal, a single ligand binds to a single receptor. However, activation of a receptor-linked enzyme can activate many copies of a component of the signaling cascade, which amplifies the signal.
Methods of Intracellular Signaling
The induction of a signaling pathway depends on the modification of a cellular component by an enzyme. There are numerous enzymatic modifications that can occur, and they are recognized in turn by the next component downstream. The following are some of the more common events in intracellular signaling.
Observe an animation of cell signaling at this site (http://openstaxcollege.org/l/cell_signals) .
Phosphorylation
One of the most common chemical modifications that occurs in signaling pathways is the addition of a phosphate group (PO4–3) to a molecule such as a protein in a process called phosphorylation. The phosphate can be added to a nucleotide such as GMP to form GDP or GTP. Phosphates are also often added to serine, threonine, and tyrosine residues of proteins, where they replace the hydroxyl group of the amino acid (Figure 9.11). The transfer of the phosphate is catalyzed by an enzyme called a kinase. Various kinases are named for the substrate they phosphorylate. Phosphorylation of serine and threonine residues often activates enzymes. Phosphorylation of tyrosine residues can either affect the activity of an enzyme or create a binding site that interacts with downstream components in the signaling cascade. Phosphorylation may activate or inactivate enzymes, and the reversal of phosphorylation, dephosphorylation by a phosphatase, will reverse the effect.
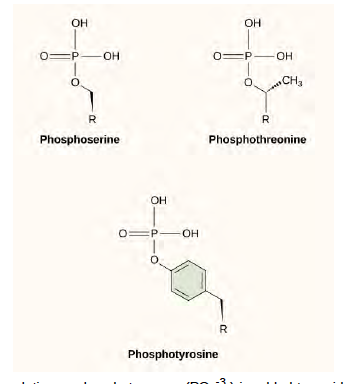
Figure 6.11 In protein phosphorylation, a phosphate group (PO4-3 ) is added to residues of the amino acids serine, threonine, and tyrosine.
Second Messengers
Second messengers are small molecules that propagate a signal after it has been initiated by the binding of the signaling molecule to the receptor. These molecules help to spread a signal through the cytoplasm by altering the behavior of certain cellular proteins.
Calcium ion is a widely used second messenger. The free concentration of calcium ions (Ca2+) within a cell is very low because ion pumps in the plasma membrane continuously use adenosine-5′-triphosphate (ATP) to remove it. For signaling purposes, Ca2+ is stored in cytoplasmic vesicles, such as the endoplasmic reticulum, or accessed from outside the cell. When signaling occurs, ligand-gated calcium ion channels allow the higher levels of Ca2+ that are present outside the cell (or in intracellular storage compartments) to flow into the cytoplasm, which raises the concentration of cytoplasmic Ca2+. The response to the increase in Ca2+ varies, depending on the cell type involved. For example, in the β-cells of the pancreas, Ca2+ signaling leads to the release of insulin, and in muscle cells, an increase in Ca2+ leads to muscle contractions.
Another second messenger utilized in many different cell types is cyclic AMP (cAMP). Cyclic AMP is synthesized by the enzyme adenylyl cyclase from ATP (Figure 6.12). The main role of cAMP in cells is to bind to and activate an enzyme called cAMP-dependent kinase (A-kinase). A-kinase regulates many vital metabolic pathways: It phosphorylates serine and threonine residues of its target proteins, activating them in the process. A-kinase is found in many different types of cells, and the target proteins in each kind of cell are different. Differences give rise to the variation of the responses to cAMP in different cells.
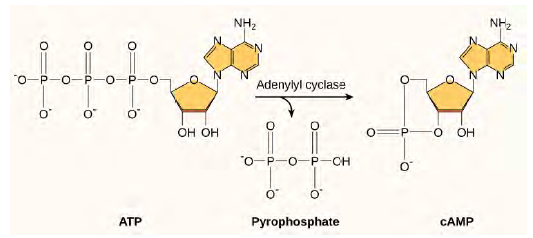
Figure 6.12 This diagram shows the mechanism for the formation of cyclic AMP (cAMP). cAMP serves as a second messenger to activate or inactivate proteins within the cell. Termination of the signal occurs when an enzyme called phosphodiesterase converts cAMP into AMP.
Present in small concentrations in the plasma membrane, inositol phospholipids are lipids that can also be converted into second messengers. Because these molecules are membrane components, they are located near membrane-bound receptors and can easily interact with them. Phosphatidylinositol (PI) is the main phospholipid that plays a role in cellular signaling. Enzymes known as kinases phosphorylate PI to form PI-phosphate (PIP) and PI-bisphosphate (PIP2).
The enzyme phospholipase C cleaves PIP2 to form diacylglycerol (DAG) and inositol triphosphate (IP3) (Figure 9.13). These products of the cleavage of PIP2 serve as second messengers. Diacylglycerol (DAG) remains in the plasma membrane and activates protein kinase C (PKC), which then phosphorylates serine and threonine residues in its target proteins. IP3 diffuses into the cytoplasm and binds to ligand-gated calcium channels in the endoplasmic reticulum to release Ca2+ that continues the signal cascade.
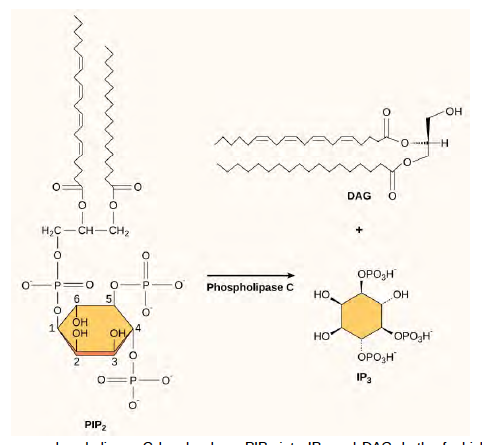
Figure 9.13 The enzyme phospholipase C breaks down PIP2 into IP3 and DAG, both of which serve as second messengers.
6.3 | Response to the Signal
Inside the cell, ligands bind to their internal receptors, allowing them to directly affect the cell’s DNA and protein-producing machinery. Using signal transduction pathways, receptors in the plasma membrane produce a variety of effects on the cell. The results of signaling pathways are extremely varied and depend on the type of cell involved as well as the external and internal conditions. A small sampling of responses is described below.
Gene Expression
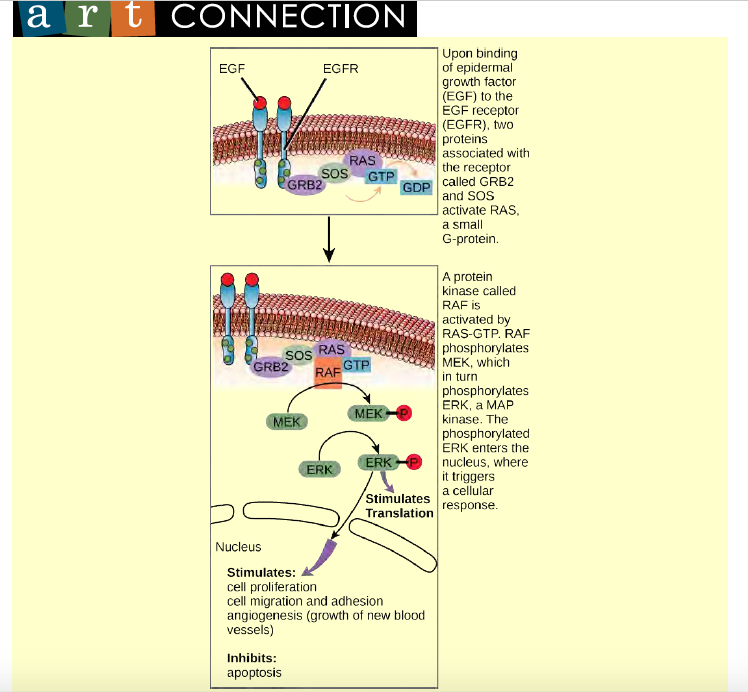
Some signal transduction pathways regulate the transcription of RNA. Others regulate the translation of proteins from mRNA. An example of a protein that regulates translation in the nucleus is the MAP kinase ERK. ERK is activated in a phosphorylation cascade when epidermal growth factor (EGF) binds the EGF receptor (see Figure 9.10). Upon phosphorylation, ERK enters the nucleus and activates a protein kinase that, in turn, regulates protein translation (Figure 6.14).
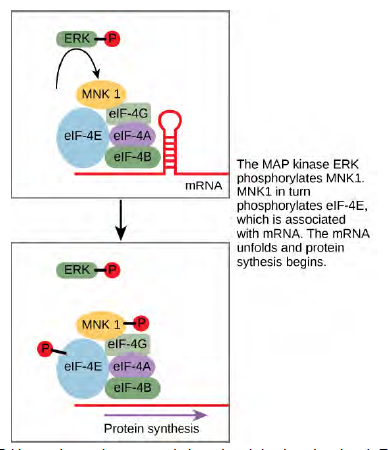
Figure 6.14 ERK is a MAP kinase that activates translation when it is phosphorylated. ERK phosphorylates MNK1, which in turn phosphorylates eIF-4E, an elongation initiation factor that, with other initiation factors, is associated with mRNA. When eIF-4E becomes phosphorylated, the mRNA unfolds, allowing protein synthesis in the nucleus to begin. (See Figure 9.10 for the phosphorylation pathway that activates ERK.)
The second kind of protein with which PKC can interact is a protein that acts as an inhibitor. An inhibitor is a molecule that binds to a protein and prevents it from functioning or reduces its function. In this case, the inhibitor is a protein called Iκ-B, which binds to the regulatory protein NF-κB. (The symbol κ represents the Greek letter kappa.) When Iκ-B is bound to NF-κB, the complex cannot enter the nucleus of the cell, but when Iκ-B is phosphorylated by PKC, it can no longer bind NF-κB, and NF-κB (a transcription factor) can enter the nucleus and initiate RNA transcription. In this case, the effect of phosphorylation is to inactivate an inhibitor and thereby activate the process of transcription.
Increase in Cellular Metabolism
The result of another signaling pathway affects muscle cells. The activation of β-adrenergic receptors in muscle cells by adrenaline leads to an increase in cyclic AMP (cAMP) inside the cell. Also known as epinephrine, adrenaline is a hormone (produced by the adrenal gland attached to the kidney) that readies the body for short-term emergencies. Cyclic AMP activates PKA (protein kinase A), which in turn phosphorylates two enzymes. The first enzyme promotes the degradation of glycogen by activating intermediate glycogen phosphorylase kinase (GPK) that in turn activates glycogen phosphorylase (GP) that catabolizes glycogen into glucose. (Recall that your body converts excess glucose to glycogen for short-term storage. When energy is needed, glycogen is quickly reconverted to glucose.) Phosphorylation of the second enzyme, glycogen synthase (GS), inhibits its ability to form glycogen from glucose. In this manner, a muscle cell obtains a ready pool of glucose by activating its formation via glycogen degradation and by inhibiting the use of glucose to form glycogen, thus preventing a futile cycle of glycogen degradation and synthesis. The glucose is then available for use by the muscle cell in response to a sudden surge of adrenaline—the “fight or flight” reflex.
Cell Growth
Cell signaling pathways also play a major role in cell division. Cells do not normally divide unless they are stimulated by signals from other cells. The ligands that promote cell growth are called growth factors. Most growth factors bind to cell-surface receptors that are linked to tyrosine kinases. These cell-surface receptors are called receptor tyrosine kinases (RTKs). Activation of RTKs initiates a signaling pathway that includes a G-protein called RAS, which activates the MAP kinase pathway described earlier. The enzyme MAP kinase then stimulates the expression of proteins that interact with other cellular components to initiate cell division.

More information on cancer biology research can be found at the National Cancer Institute website (http://www.cancer.gov/cancertopics/understandingcancer/targetedtherapies).
Cell Death
When a cell is damaged, superfluous, or potentially dangerous to an organism, a cell can initiate a mechanism to trigger programmed cell death, or apoptosis. Apoptosis allows a cell to die in a controlled manner that prevents the release of potentially damaging molecules from inside the cell. There are many internal checkpoints that monitor a cell’s health; if abnormalities are observed, a cell can spontaneously initiate the process of apoptosis. However, in some cases, such as a viral infection or uncontrolled cell division due to cancer, the cell’s normal checks and balances fail. External signaling can also initiate apoptosis. For example, most normal animal cells have receptors that interact with the extracellular matrix, a network of glycoproteins that provides structural support for cells in an organism. The binding of cellular receptors to the extracellular matrix initiates a signaling cascade within the cell. However, if the cell moves away from the extracellular matrix, the signaling ceases, and the cell undergoes apoptosis. This system keeps cells from traveling through the body and proliferating out of control, as happens with tumor cells that metastasize.
Another example of external signaling that leads to apoptosis occurs in T-cell development. T-cells are immune cells that bind to foreign macromolecules and particles, and target them for destruction by the immune system. Normally, T-cells do not target “self” proteins (those of their own organism), a process that can lead to autoimmune diseases. In order to develop the ability to discriminate between self and non-self, immature T-cells undergo screening to determine whether they bind to so-called self proteins. If the T-cell receptor binds to self proteins, the cell initiates apoptosis to remove the potentially dangerous cell.
Apoptosis is also essential for normal embryological development. In vertebrates, for example, early stages of development include the formation of web-like tissue between individual fingers and toes (Figure 9.15). During the course of normal development, these unneeded cells must be eliminated, enabling fully separated fingers and toes to form. A cell signaling mechanism triggers apoptosis, which destroys the cells between the developing digits.
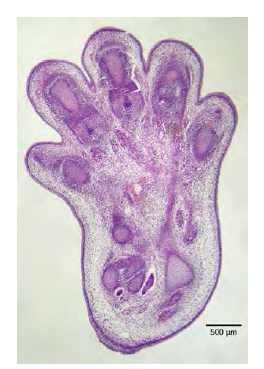
Figure 6.15 The histological section of a foot of a 15-day-old mouse embryo, visualized using light microscopy, reveals areas of tissue between the toes, which apoptosis will eliminate before the mouse reaches its full gestational age at 27 days. (credit: modification of work by Michal Mañas)
Termination of the Signal Cascade
The aberrant signaling often seen in tumor cells is proof that the termination of a signal at the appropriate time can be just as important as the initiation of a signal. One method of stopping a specific signal is to degrade the ligand or remove it so that it can no longer access its receptor. One reason that hydrophobic hormones like estrogen and testosterone trigger long- lasting events is because they bind carrier proteins. These proteins allow the insoluble molecules to be soluble in blood, but they also protect the hormones from degradation by circulating enzymes.
Inside the cell, many different enzymes reverse the cellular modifications that result from signaling cascades. For example, phosphatases are enzymes that remove the phosphate group attached to proteins by kinases in a process called dephosphorylation. Cyclic AMP (cAMP) is degraded into AMP by phosphodiesterase, and the release of calcium stores is reversed by the Ca2+ pumps that are located in the external and internal membranes of the cell.
6.4 | Signaling in Single-Celled Organisms
Within-cell signaling allows bacteria to respond to environmental cues, such as nutrient levels, some single-celled organisms also release molecules to signal to each other.
Signaling in Yeast
Yeasts are eukaryotes (fungi), and the components and processes found in yeast signals are similar to those of cell-surface receptor signals in multicellular organisms. Budding yeasts (Figure 6.16) are able to participate in a process that is similar to sexual reproduction that entails two haploid cells (cells with one-half the normal number of chromosomes) combining to form a diploid cell (a cell with two sets of each chromosome, which is what normal body cells contain). In order to find another haploid yeast cell that is prepared to mate, budding yeasts secrete a signaling molecule called mating factor. When mating factor binds to cell-surface receptors in other yeast cells that are nearby, they stop their normal growth cycles and initiate a cell signaling cascade that includes protein kinases and GTP-binding proteins that are similar to G-proteins.

Figure 6.16 Budding Saccharomyces cerevisiae yeast cells can communicate by releasing a signaling molecule called mating factor. In this micrograph, they are visualized using differential interference contrast microscopy, a light microscopy technique that enhances the contrast of the sample.
Signaling in Bacteria
Signaling in bacteria enables bacteria to monitor extracellular conditions, ensure that there are sufficient amounts of nutrients, and ensure that hazardous situations are avoided. There are circumstances, however, when bacteria communicate with each other.
The first evidence of bacterial communication was observed in a bacterium that has a symbiotic relationship with Hawaiian bobtail squid. When the population density of the bacteria reaches a certain level, specific gene expression is initiated, and the bacteria produce bioluminescent proteins that emit light. Because the number of cells present in the environment (cell density) is the determining factor for signaling, bacterial signaling was named quorum sensing. In politics and business, a quorum is the minimum number of members required to be present to vote on an issue.
Quorum sensing uses autoinducers as signaling molecules. Autoinducers are signaling molecules secreted by bacteria to communicate with other bacteria of the same kind. The secreted autoinducers can be small, hydrophobic molecules such as acyl-homoserine lactone, (AHL) or larger peptide-based molecules; each type of molecule has a different mode of action. When AHL enters target bacteria, it binds to transcription factors, which then switch gene expression on or off (Figure 9.17). The peptide autoinducers stimulate more complicated signaling pathways that include bacterial kinases. The changes in bacteria following exposure to autoinducers can be quite extensive. The pathogenic bacterium Pseudomonas aeruginosa has 616 different genes that respond to autoinducers.
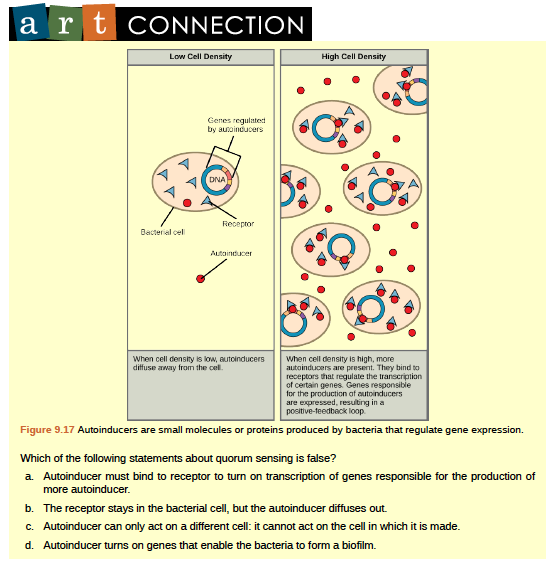
Some species of bacteria that use quorum sensing form biofilms, complex colonies of bacteria (often containing several species) that exchange chemical signals to coordinate the release of toxins that will attack the host. Bacterial biofilms (Figure 9.18) can sometimes be found on medical equipment; when biofilms invade implants such as hip or knee replacements or heart pacemakers, they can cause life-threatening infections.
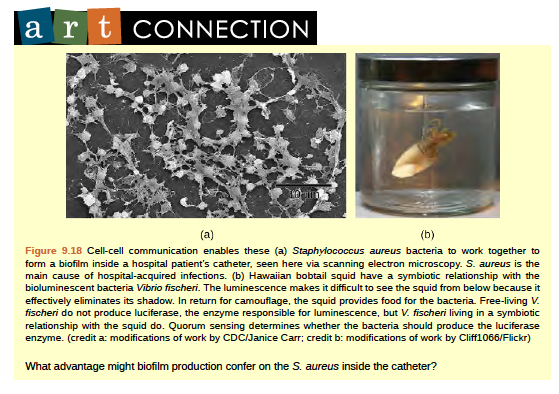
Figure 6.18 Cell-cell communication enables these (a) Staphylococcus aureus bacteria to work together to form a biofilm inside a hospital patient’s catheter, seen here via scanning electron microscopy. S. aureus is the main cause of hospital-acquired infections. (b) Hawaiian bobtail squid have a symbiotic relationship with the bioluminescent bacteria Vibrio fischeri. The luminescence makes it difficult to see the squid from below because it effectively eliminates its shadow. In return for camouflage, the squid provides food for the bacteria. Free-living V. fischeri do not produce luciferase, the enzyme responsible for luminescence, but V. fischeri living in a symbiotic relationship with the squid do. Quorum sensing determines whether the bacteria should produce the luciferase enzyme. (credit a: modifications of work by CDC/Janice Carr; credit b: modifications of work by Cliff1066/Flickr)
Research on the details of quorum sensing has led to advances in growing bacteria for industrial purposes. Recent discoveries suggest that it may be possible to exploit bacterial signaling pathways to control bacterial growth; this process could replace or supplement antibiotics that are no longer effective in certain situations.
Watch geneticist Bonnie Bassler discuss her discovery (http://openstaxcollege.org/l/bacteria_talk) of quorum sensing in biofilm bacteria in squid.
2. G. Manning, G.D. Plowman, T. Hunter, S. Sudarsanam, “Evolution of Protein Kinase Signaling from Yeast to Man,” Trends in Biochemical Sciences 27, no. 10 (2002): 514–520.
Watch this collection (http://openstaxcollege.org/l/bacteria_biofilm) of interview clips with biofilm researchers in “What Are Bacterial Biofilms?”
KEY TERMS
apoptosis programmed cell death
autocrine signal signal that is sent and received by the same or similar nearby cells
autoinducer signaling molecule secreted by bacteria to communicate with other bacteria of its kind and others
cell-surface receptor cell-surface protein that transmits a signal from the exterior of the cell to the interior, even though the ligand does not enter the cell
chemical synapse small space between axon terminals and dendrites of nerve cells where neurotransmitters function cyclic AMP (cAMP) second messenger that is derived from ATP
cyclic AMP-dependent kinase (also, protein kinase A, or PKA) kinase that is activated by binding to cAMP diacylglycerol (DAG) cleavage product of PIP2 that is used for signaling within the plasma membrane
dimer chemical compound formed when two molecules join together
dimerization (of receptor proteins) interaction of two receptor proteins to form a functional complex called a dimer
endocrine cell cell that releases ligands involved in endocrine signaling (hormones)
endocrine signal long-distance signal that is delivered by ligands (hormones) traveling through an organisms circulatory system from the signaling cell to the target cell
enzyme-linked receptor cell-surface receptor with intracellular domains that are associated with membrane-bound enzymes
extracellular domain region of a cell-surface receptor that is located on the cell surface
G-protein-linked receptor cell-surface receptor that activates membrane-bound G-proteins to transmit a signal from the receptor to nearby membrane components
growth factor ligand that binds to cell-surface receptors and stimulates cell growth
inhibitor molecule that binds to a protein (usually an enzyme) and keeps it from functioning
inositol phospholipid lipid present at small concentrations in the plasma membrane that is converted into a second messenger; it has inositol (a carbohydrate) as its hydrophilic head group
inositol triphosphate (IP3) cleavage product of PIP2 that is used for signaling within the cell
intercellular signaling communication between cells
internal receptor (also, intracellular receptor) receptor protein that is located in the cytosol of a cell and binds to ligands that pass through the plasma membrane
intracellular signaling communication within cells
ion channel-linked receptor cell-surface receptor that forms a plasma membrane channel, which opens when a ligand binds to the extracellular domain (ligand-gated channels)
kinase enzyme that catalyzes the transfer of a phosphate group from ATP to another molecule
ligand molecule produced by a signaling cell that binds with a specific receptor, delivering a signal in the process
mating factor signaling molecule secreted by yeast cells to communicate to nearby yeast cells that they are available to mate and communicating their mating orientation
neurotransmitter chemical ligand that carries a signal from one nerve cell to the next
paracrine signal signal between nearby cells that is delivered by ligands traveling in the liquid medium in the space between the cells
phosphatase enzyme that removes the phosphate group from a molecule that has been previously phosphorylated
phosphodiesterase enzyme that degrades cAMP, producing AMP, to terminate signaling
quorum sensing method of cellular communication used by bacteria that informs them of the abundance of similar (or different) bacteria in the environment
receptor protein in or on a target cell that bind to ligands
second messenger small, non-protein molecule that propagates a signal within the cell after activation of a receptor causes its release
signal integration interaction of signals from two or more different cell-surface receptors that merge to activate the same response in the cell
signal transduction propagation of the signal through the cytoplasm (and sometimes also the nucleus) of the cell
signaling cell cell that releases signal molecules that allow communication with another cell
signaling pathway (also signaling cascade) chain of events that occurs in the cytoplasm of the cell to propagate the signal from the plasma membrane to produce a response
synaptic signal chemical signal (neurotransmitter) that travels between nerve cells target cell cell that has a receptor for a signal or ligand from a signaling cell
CHAPTER SUMMARY
6.1 Signaling Molecules and Cellular Receptors
Cells communicate by both inter- and intracellular signaling. Signaling cells secrete ligands that bind to target cells and initiate a chain of events within the target cell. The four categories of signaling in multicellular organisms are paracrine signaling, endocrine signaling, autocrine signaling, and direct signaling across gap junctions. Paracrine signaling takes place over short distances. Endocrine signals are carried long distances through the bloodstream by hormones, and autocrine signals are received by the same cell that sent the signal or other nearby cells of the same kind. Gap junctions allow small molecules, including signaling molecules, to flow between neighboring cells.
Internal receptors are found in the cell cytoplasm. Here, they bind ligand molecules that cross the plasma membrane; these receptor-ligand complexes move to the nucleus and interact directly with cellular DNA. Cell-surface receptors transmit a signal from outside the cell to the cytoplasm. Ion channel-linked receptors, when bound to their ligands, form a pore through the plasma membrane through which certain ions can pass. G-protein-linked receptors interact with a G-protein on the cytoplasmic side of the plasma membrane, promoting the exchange of bound GDP for GTP and interacting with other enzymes or ion channels to transmit a signal. Enzyme-linked receptors transmit a signal from outside the cell to an intracellular domain of a membrane-bound enzyme. Ligand binding causes activation of the enzyme. Small hydrophobic ligands (like steroids) are able to penetrate the plasma membrane and bind to internal receptors. Water-soluble hydrophilic ligands are unable to pass through the membrane; instead, they bind to cell-surface receptors, which transmit the signal to the inside of the cell.
6.2 Propagation of the Signal
Ligand binding to the receptor allows for signal transduction through the cell. The chain of events that conveys the signal through the cell is called a signaling pathway or cascade. Signaling pathways are often very complex because of the interplay between different proteins. A major component of cell signaling cascades is the phosphorylation of molecules by enzymes known as kinases. Phosphorylation adds a phosphate group to serine, threonine, and tyrosine residues in a protein, changing their shapes, and activating or inactivating the protein. Small molecules like nucleotides can also be phosphorylated. Second messengers are small, non-protein molecules that are used to transmit a signal within a cell. Some examples of second messengers are calcium ions (Ca2+), cyclic AMP (cAMP), diacylglycerol (DAG), and inositol triphosphate (IP3).
6.3 Response to the Signal
The initiation of a signaling pathway is a response to external stimuli. This response can take many different forms, including protein synthesis, a change in the cell’s metabolism, cell growth, or even cell death. Many pathways influence the cell by initiating gene expression, and the methods utilized are quite numerous. Some pathways activate enzymes that interact with DNA transcription factors. Others modify proteins and induce them to change their location in the cell. Depending on the status of the organism, cells can respond by storing energy as glycogen or fat, or making it available in the form of glucose. A signal transduction pathway allows muscle cells to respond to immediate requirements for energy in the form of glucose. Cell growth is almost always stimulated by external signals called growth factors. Uncontrolled cell growth leads to cancer, and mutations in the genes encoding protein components of signaling pathways are often found in tumor cells. Programmed cell death, or apoptosis, is important for removing damaged or unnecessary cells. The use of cellular signaling to organize the dismantling of a cell ensures that harmful molecules from the cytoplasm are not released into the spaces between cells, as they are in uncontrolled death, necrosis. Apoptosis also ensures the efficient recycling of the components of the dead cell. Termination of the cellular signaling cascade is very important so that the response to a signal is appropriate in both timing and intensity. Degradation of signaling molecules and dephosphorylation of phosphorylated intermediates of the pathway by phosphatases are two ways to terminate signals within the cell.
6.4 Signaling in Single-Celled Organisms
Yeasts and multicellular organisms have similar signaling mechanisms. Yeasts use cell-surface receptors and signaling cascades to communicate information on mating with other yeast cells. The signaling molecule secreted by yeasts is called mating factor.
Bacterial signaling is called quorum sensing. Bacteria secrete signaling molecules called autoinducers that are either small, hydrophobic molecules or peptide-based signals. The hydrophobic autoinducers, such as AHL, bind transcription factors and directly affect gene expression. The peptide-based molecules bind kinases and initiate signaling cascades in the cells.
Review Questions:
How does NF-κB induce gene expression?
a. A small, hydrophobic ligand binds to NF-κB, activating it.
b. Phosphorylation of the inhibitor Iκ-B dissociates the complex between it and NF-κB, and allows NF-κB to enter the nucleus and stimulate transcription.
c. NF-κB is phosphorylated and is then free to enter the nucleus and bind DNA.
d. NF-κB is a kinase that phosphorylates a transcription factor that binds DNA and promotes protein production.
Apoptosis can occur in a cell when the cell is ________________.
a. damaged
b. no longer needed
c. infected by a virus
d. all of the above
What is the effect of an inhibitor binding an enzyme?
a. The enzyme is degraded.
b. The enzyme is activated.
c. The enzyme is inactivated.
d. The complex is transported out of the cell.
Which type of molecule acts as a signaling molecule in yeasts?
a. steroid
b. autoinducer
c. mating factor
d. second messenger
Quorum sensing is triggered to begin when ___________.
a. treatment with antibiotics occurs
b. bacteria release growth hormones
c. bacterial protein expression is switched on
d. a sufficient number of bacteria are present
CRITICAL THINKING QUESTIONS
What is the difference between intracellular signaling and intercellular signaling?
How are the effects of paracrine signaling limited to an area near the signaling cells?
The same second messengers are used in many different cells, but the response to second messengers is different in each cell. How is this possible?
What would happen if the intracellular domain of a cell-surface receptor was switched with the domain from another receptor?
What is a possible result of a mutation in a kinase that controls a pathway that stimulates cell growth?
How does the extracellular matrix control the growth of cells?
What characteristics make yeasts a good model for learning about signaling in humans?
Why is signaling in multicellular organisms more complicated than signaling in single-celled organisms?
Adapted from:
OpenStax, Biology. OpenStax. May 20, 2013. <http://cnx.org/content/col11448/latest/>
“Download for free at http://cnx.org/content/col11448/latest/.”

