2.1 PreLab Reading – Cell Metabolism
Cell Metabolism
Food undergoes a transformative journey within our bodies, requiring both mechanical and chemical processes to convert it into absorbable forms for energy and building blocks. This complex process is driven by specialized enzymes that facilitate chemical digestion. In this lab, you will study posters detailing key metabolic pathways in chemical digestion such as gluconeogenesis, glycogenesis, glycogenolysis, proteolysis, lipolysis, and lipogenesis to understand how our bodies manage energy storage and utilization from different biomolecules.
To comprehend how this works, let’s first establish some foundational vocabulary:
- Digestion: The intricate process of breaking down food into nutrients capable of transport into the body.
- Digestive enzymes: Specialized proteins synthesized within specific cells, deployed into the digestive tract to expedite the extracellular breakdown of biological nutrients, including proteins, carbohydrates, and lipids.
- Mechanical digestion: The physical manipulation of food, encompassing actions like chewing, mixing, and peristalsis.
Metabolism is the sum of all chemical reactions in the human body and is classified into two categories: catabolic and anabolic. A metabolic pathway is a series of chemical reactions that transform molecules step by step through metabolic intermediates into a final product or products.
Anabolic pathways require energy to build complex molecules from simpler building blocks, while catabolic pathways release energy by breaking down complex molecules into simpler components. In other words, catabolic reactions degrade large molecules, whereas anabolic reactions use energy to synthesize larger, more complex molecules. For nutrients from ingested food to be absorbed, digestion must first occur, breaking down into easily absorbable units. This process involves both mechanical actions such as chewing and peristalsis, and chemical actions carried by digestive enzymes.
Digestive enzymes are synthesized within specialized cells and released into the digestive tract, where they accelerate the extracellular breakdown of nutrients in food and fluids. The products of chemical digestion are then absorbed into the bloodstream or lymphatic vessels of the small intestine, transported to cells throughout the body, and utilized in intracellular metabolism. Key metabolic processes include aerobic respiration, glycolysis, lipolysis, and protein synthesis.
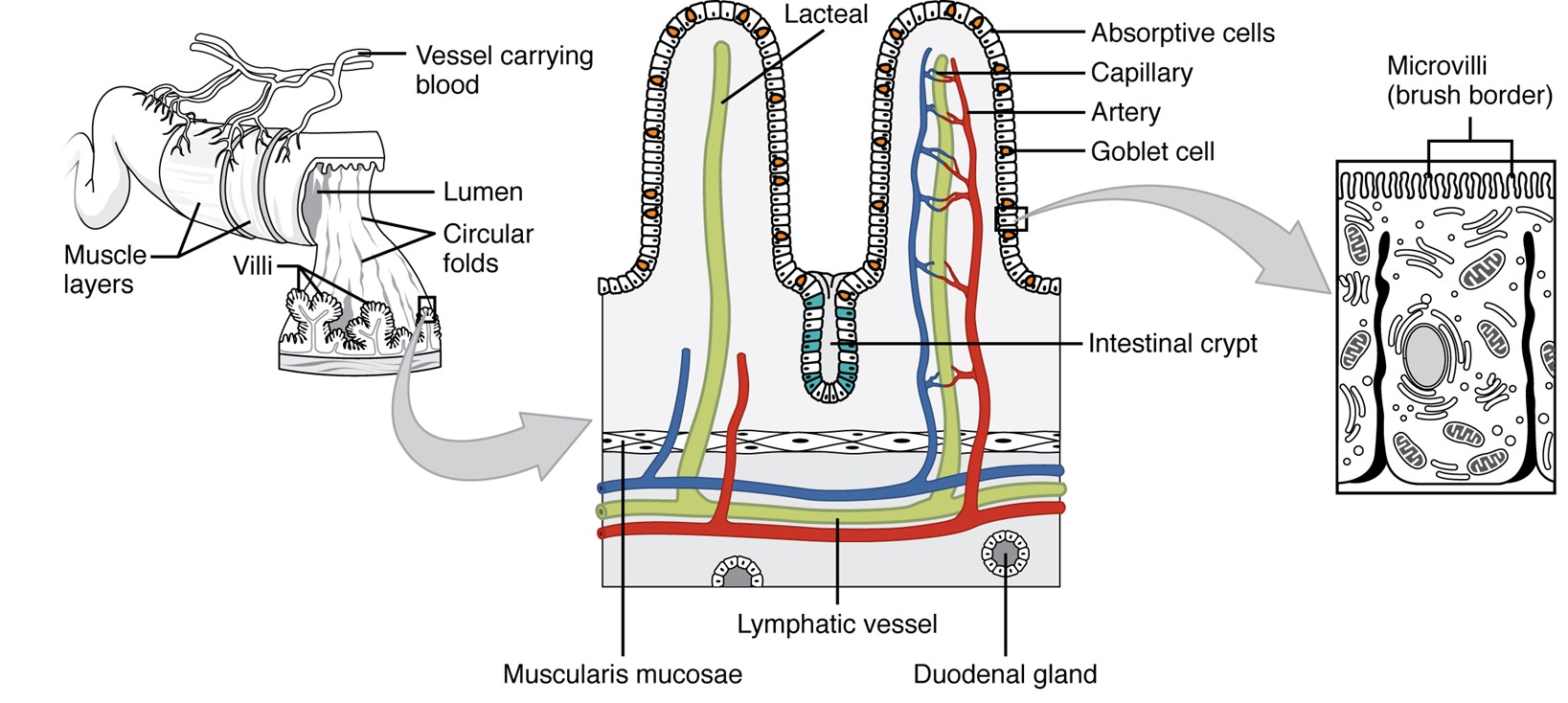
The absorptive surface of the small intestine. It illustrates the structural features that increase surface area for nutrient absorption. The inner lining is shown with folds called circular folds. These folds are covered with finger-like projections called villi, and each villus is lined with even smaller hair-like structures called microvilli. Together, the folds, villi, and microvilli create a large surface area that allows efficient absorption of nutrients into the bloodstream and lymphatic system.
(Figure by OpenStax, used under a Creative Commons Attribution license.)
Chemical actions depend on enzyme-catalyzed hydrolysis, in which hydrolytic enzymes, or hydrolases, add water to break down larger food molecules into their individual building blocks.
Proteins + H20 + enzyme ⟶ amino acids + enzyme
Fats + H20 + enzyme ⟶ fatty acids + glycerol (or monoglyceride) + enzyme
Carbohydrates + H20 + enzyme ⟶ monosaccharides + enzyme
Each of these reactions within the body depends on the presence and activity of specific hydrolytic enzymes. Factors that affect enzyme activity include pH and temperature.
Temperature and pH are tightly regulated within narrow ranges in the body to ensure optimal conditions for enzymatic activity. These organic molecules are essential in maintaining the body’s metabolic balance. During the absorptive state (3-5 after eating), or fed, state, glucose serves as the primary energy source, with minimal use of absorbed fats and amino acids. In contrast, the postabsorptive (8-12 after a meal), or fasting, state prompts the body to rely on its energy reserves to fulfill its energy demands.
Proteins
Proteins also known as polypeptides are composed of amino acids, monomers covalently linked by peptide bonds. All amino acids share a common chemical backbone but differ in their R groups (side chains), which determine whether they are classified as polar, charged, or nonpolar. Although all proteins have hydrophilic (water attracting) properties, the degree of hydrophilicity varies. Some proteins feature a higher proportion of nonpolar side chains, making them less hydrophilic compared to others.
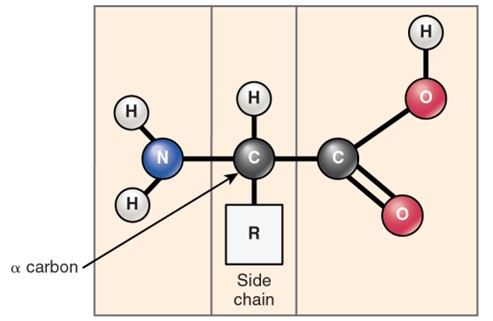
The general structure of an amino acid. It shows a central carbon atom (the alpha carbon) bonded to four groups: an amino group, a carboxyl group, a hydrogen atom, a variable side chain (R group) which determines the identity and properties of the amino acid. The diagram highlights that all amino acids have this basic structure, with the R group being the component that differs among them.
(Figure by OpenStax, used under a Creative Commons Attribution license.)
Proteins possess complex structures, transitioning from a primary to secondary, tertiary, and often quaternary structure to fulfill specific functions. Maintaining the optimal temperature is crucial for preserving their structure and ensuring proper enzyme activity.
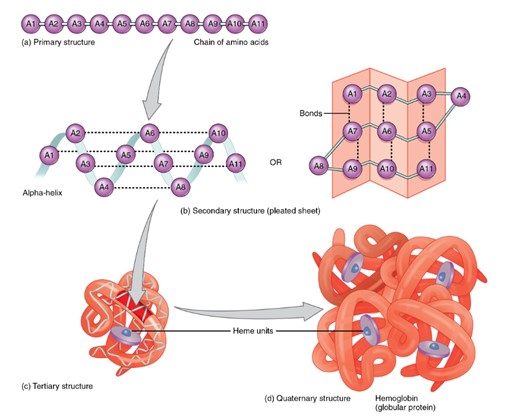
The four levels of protein structure:
a. Primary structure: a linear sequence of amino acids linked by peptide bonds.
b. Secondary structure: local folding of the amino acid chain into structures such as alpha helices and beta-pleated sheets, stabilized by hydrogen bonds.
c. Tertiary structure: the overall three-dimensional folding of a single polypeptide chain, formed by interactions between the side chains (R groups), including hydrogen bonds, ionic bonds, and hydrophobic interactions.
d. Quaternary structure: the assembly of multiple polypeptide chains (subunits) into a functional protein complex.
The diagram visually shows each level, progressing from a simple linear chain to a complex multi-subunit protein.
(Figure by OpenStax, used under a Creative Commons Attribution license.)
Protein Digestion
Protein digestion begins in the stomach with the enzyme pepsin. Inactive when the stomach is empty, pepsin is secreted by the chief cells in its inactive form, pepsinogen. Upon food entry, stomach acid (HCL) and active pepsin molecules convert pepsinogen into pepsin. Pepsin hydrolyzes proteins into smaller peptides and functions optimally at the stomach’s acidic pH (around 2). As chyme moves into the small intestine, where the pH rises to approximately 9, pepsin becomes denatured.
In the duodenum, pancreatic enzymes trypsin, chymotrypsin, and carboxypeptidase continue breaking down peptides into smaller fragments. Enzymes produced in the brush border of the small intestine, including aminopeptidases and dipeptidases, complete the process of hydrolyzing peptides into individual amino acids.
After digestion, amino acids are absorbed into the capillaries of the small intestine and transported to the liver via the hepatic portal vein.
Protein Metabolism
Cells synthesize proteins through proteogenesis (the processes of transcription and translation) and break down through proteolysis when they are no longer needed. Cells are efficient and recycle amino acids whenever possible, reusing them for new protein synthesis.
The rates of proteogenesis and proteolysis vary depending on the body’s metabolic state:
- Absorptive State (Fed State): After eating, when energy and nutrients are abundant, proteogenesis predominates. Amino acids absorbed from digestion are primarily used for protein synthesis, tissue repair, and enzyme production, while excess amino acids may be converted to other compounds or store as fat.
- Post-Absorptive State (Fasting State): During fasting, when blood glucose levels drop and glycogen stores become depleted, the body shifts to catabolic processes. Proteolysis increases, breaking down muscle and other tissue proteins to release amino acids. These amino acids can be used for gluconeogenesis in the liver to maintain blood glucose levels or, if necessary, deaminated and used in aerobic respiration for energy. This reliance on amino acids for energy is not ideal, as it stresses the liver and kidneys over time.
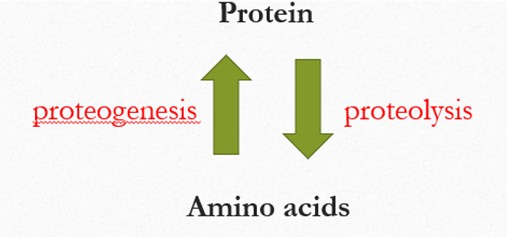
The relationship between proteins and amino acids. There are two green arrows pointing in opposite direction between the words “Protein” at the top and “Amino Acids” at the bottom. The upward pointing arrow, labeled proteogenesis in red, represents the synthesis of proteins from amino acids. The downward-pointing arrow, labeled proteolysis in red, represents the breakdown of proteins into amino acids. This diagram illustrates the dynamic process of protein formation and degradation in the body.
Carbohydrates
Carbohydrates, also known as polysaccharides, are polymers of simple sugars (monosaccharides) linked by glycosidic bonds. Among these, glucose is the primary substrate for aerobic respiration and also plays a key role in fermentation. While most carbohydrates function as energy sources, some serve specialized structural roles, such as forming cell surface coatings.
The abundance of hydroxyl, ketone, and aldehyde groups on simple sugars makes all carbohydrates hydrophilic to varying degrees.
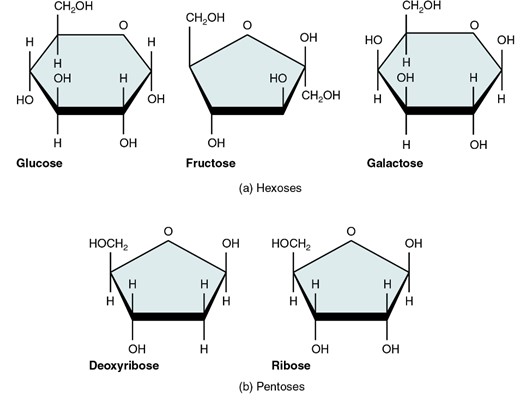
Five monosaccharides important in the human body. Each monosaccharide is shown as a simple chemical structure with its name underneath. The monosaccharide included are: glucose, fructose, galactose, ribose and deoxyribose. The image illustrates the ring or chain forms of each molecule, highlighting the basic carbon, hydrogen, and oxygen arrangements.
(Figure by OpenStax, used under a Creative Commons Attribution license.)
Digestion and Absorption
Carbohydrate digestion begins with amylase, an enzyme that breaks down starch (a major dietary carbohydrate).
- Salivary amylase, secreted by the salivary glands, initiates carbohydrate digestion in the mouth.
- Pancreatic amylase, released into the duodenum, continues this process.
As carbohydrates are hydrolyzed into monosaccharides, they become absorbable. Absorption occurs primarily in the small intestine, though small amounts may occur in the mouth. It is important to note that carbohydrates are not inherently “bad”; like all nutrients, excessive consumption can have negative effects. However, they are essential for homeostasis, as glucose is the body’s preferred energy source, readily converted into ATP.
One of the most important polysaccharides in human nutrition is amylose, a component of starch.
- Amylose is hydrolyzed by amylase into maltose (a disaccharide).
- Maltose is then broken down into two glucose molecules by maltase, an enzyme located in the brush border of the small intestine.
- Glucose is absorbed into the bloodstream via the hepatic portal vein and transported to the liver.
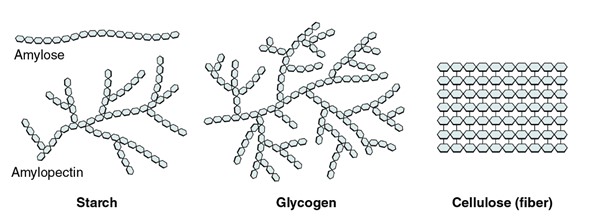
Three important polysaccharides: starch, glycogen, and cellulose. Each is illustrated as a chain of repeating glucose units, but with different branching patterns. Starch is shown as a mostly linear chain with occasional branches. Glycogen appears as a highly branched structure. Cellulose is depicted as a straight, unbranched chain of glucose molecules linked tightly together.
(Figure by OpenStax, used under a Creative Commons Attribution license.)
In the liver, glucose may:
- Be converted into ATP for immediate energy.
- Be stored as glycogen, a branched polymer of alpha-glucose.
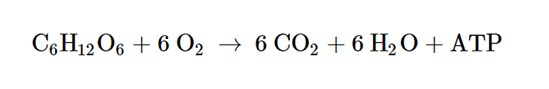
The chemical equation for cellular respiration. This represents glucose reacting with oxygen to produce carbon dioxide, water, and ATP (energy).
Carbohydrate Metabolism
The primary role of carbohydrates is the energy production through aerobic respiration or, under anaerobic conditions, fermentation. Carbohydrate metabolism alternates between two physiological states:
- Absorptive State (Fed State): Occurs after eating when nutrients are absorbed and stored. Glucose is stored as glycogen in the liver and skeletal muscles through glycogenesis.
- Post-Absorptive State (Fasting State): Occurs after prolonged periods without food, when stored nutrients are mobilized.
- The liver initiates glycogenolysis, breaking down glycogen into glucose for release into the blood.
- Muscle cells also perform glycogenolysis but retain the glucose for their own aerobic respiration.
- When glycogen stores are depleted, the liver initiates gluconeogenesis, synthesizing glucose from non-carbohydrate sources such as amino acids and lipids.
The breakdown of glucose into ATP occurs via glycolysis (catabolic), whereas glycogenesis (anabolic) forms glycogen from glucose. Glycogenolysis (catabolic) releases glucose from glycogen, and gluconeogenesis synthesizes new glucose when other stores are low.
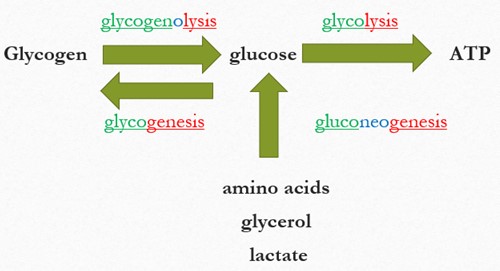
A simple flow diagram showing how glucose is produced and used in the body. Glycogen is shown on the left, with an arrow pointing from glycogen to glucose labeled glycogenolysis. Another arrow points from glucose back to glycogen labeled glycogenesis. From glucose, a large arrow points to the right toward ATP, labeled glycolysis.
Below glucose, an upward arrow shows that amino acids, glycerol, and lactate can be converted into glucose through a process labeled gluconeogenesis. The diagram illustrates the major metabolic pathways that create or use glucose.
Two key pancreatic hormones regulate carbohydrate metabolism:
- Insulin: Lowers blood glucose by promoting glucose uptake into cells, stimulating glycogenesis, and inhibiting glycogenolysis and gluconeogenesis.
- Glucagon: Raises blood glucose by stimulating glycogenolysis and gluconeogenesis while inhibiting glycogenesis.
Lipids
Lipids are primarily composed of fats and fatty acids, which serve as their monomers and are linked by simple covalent bonds, often in the form of ester bonds. Unlike carbohydrates and proteins, lipids are predominantly hydrophobic, a property that significantly influences their roles in physiology.
In human physiology, lipids play diverse roles:
- Energy storage (triglycerides in adipocytes)
- Structural components (phospholipids in cell membranes)
- Cell signaling and hormone synthesis (steroids, eicosanoids, and cholesterol)
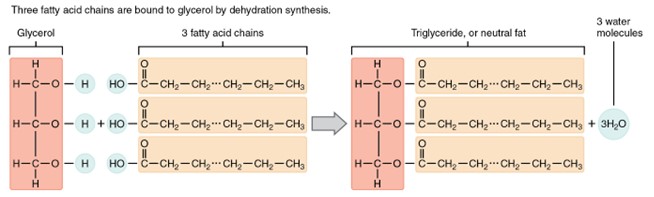
The structure of a triglyceride. It includes one glycerol molecule on the left, represented as a three-carbon backbone, with three long fatty acids chains extending to the right. Each fatty acid is attached to one of the glycerol carbons. The figure demonstrates that triglycerides are made of one glycerol molecule bonded to three fatty acids.
(Figure by OpenStax, used under a Creative Commons Attribution license.)
Lipid Digestion
Because lipids are insoluble in water, they require emulsification before digestion. Bile salts, produced by the liver, stored in the gallbladder, and secreted into the small intestine, break large fat globules (mainly triglycerides) into smaller micelles. This increases surface area for enzymatic action.
- Pancreatic lipases hydrolyze triglycerides into fatty acids and monoglycerides.
- These smaller molecules diffuse into the epithelial cells of the small intestine, where they are reassembled into triglycerides.
- Triglycerides are then packaged into chylomicrons and transported through the lymphatic system before entering the bloodstream for distribution.
In addition to transporting fatty acids and triglycerides, micelles also facilitate the absorption for fat-soluble vitamins (A, D, E, and K). Micelles are spherical structures formed by phospholipids arranged with their hydrophilic (water-attracting) heads facing outward and hydrophobic (water-repelling) tails inward. This amphipathic arrangement enables them to transport nonpolar molecules efficiently through the aqueous environment of the intestine. phospholipids that are arranged in a way they make up a spherical shape and characterized by their amphipathic property which helps in the transport of non-polar molecules.

A micelle, represented as a spherical structure made of a single layer of phospholipids. Each phospholipid is drawn with a round “head” facing outward toward the surrounding water and a pair of “tails” pointing inward toward the center of the sphere. The arrangement illustrates how phospholipids self-assemble so that their hydrophilic heads face the aqueous environment while their hydrophobic tails cluster together inside.
(Figure by Wikimedia, used under Creative Commons Attribution license.)
Lipids Metabolism
The synthesis of lipids is known as lipogenesis, while their breakdown is called lipolysis. Both processes fluctuate depending on metabolic state:
- Absorptive State (Fed State): When nutrient availability is high, lipogenesis dominates. Adipocytes are highly active in storing energy by converting excess nutrients, including carbohydrates and proteins, into triglycerides for future use.
- Post-Absorptive State (Fasting State): During fasting or low carbohydrate availability, lipolysis becomes more active. Triglycerides store in adipocytes are broken down into glycerol and fatty acids, which are used in aerobic respiration to produce ATP.
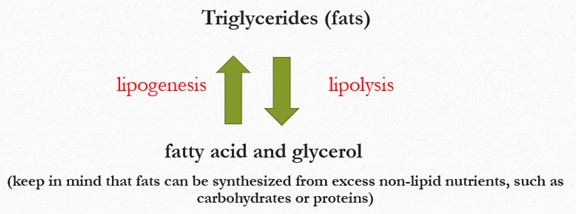
A simple diagram showing how triglycerides (fats) are broken down and formed. At the top, the label read “Triglycerides (fats)”. Below it, a downward arrow points to “fatty acid and glycerol”, labeled lipolysis to indicate the breakdown of triglycerides. A second arrow pointing upward from “fatty acid and glycerol” back to “Triglycerides (fats)” is labeled lipogenesis, indicating fat synthesis. At the bottom, a note explains that fats can also synthesized from excess non-lipid nutrients, such as carbohydrates or proteins.
If triglycerides are broken down faster than they are used, the liver converts fatty acids into ketones, which can serve as alternative energy source, even for neurons. However, excessive ketone production can lead to ketoacidosis, a dangerous and potentially life-threatening condition. (Note: This is why prolonged high-fat, low-carb “keto diets” can have serious health consequences)
Cholesterol: Function and Clinical Importance
Cholesterol is a lipid with crucial roles in human physiology:
- Structural component of cell membranes (helping maintain fluidity)
- Precursor for steroid hormones (e.g., cortisol, estrogen, testosterone)
- Required for bile salt and vitamin D synthesis
However, cholesterol transport in the blood is a key indicator of cardiovascular health. Because cholesterol is hydrophobic, it is carried by lipoproteins:
Key Blood Lipid Measurements:
- Total Cholesterol: The overall amount of cholesterol in the blood (HDL + LDL +VLDL components). High total cholesterol increases cardiovascular risk, but interpretation requires looking at individual components.
- LDL Cholesterol (Bad Cholesterol): Carries cholesterol to tissues. Excess LDL contributes to plaque formation (atherosclerosis), narrowing arteries and increasing heart attack or stroke risk. Lower LDL is better.
- HDL Cholesterol (Good Cholesterol): Transports excess cholesterol from tissues back to the liver for excretion. Higher HDL is protective against heart disease.
- Total Cholesterol/HDL ratio: Gives a quick risk estimate.
- Triglycerides: Measure of circulating fats used for energy storage. High triglycerides are linked to metabolic syndrome and increased cardiovascular risk.
- LDL/HDL Ratio: Another risk marker, lower ratios indicate a healthier balance of “good” vs “bad” cholesterol.
- Non-HDL Cholesterol: Total cholesterol minus HDL; represents all “bad cholesterol (LDL + VLDL). It is considered a better predictor of cardiovascular risk than total cholesterol alone.
This week in lab, you will measure several values and evaluate what they reveal about your current health status. To help you prepare, please watch the following training video before coming to lab: https://prod.ptsdiagnostics.com/cardiochek-plus-v-12-smart-bundle-training/
The video will guide you through:
- Proper use of the CardioChek device
- Correct fingerstick technique
- Required cleaning and disinfecting procedures
Watching the video ahead of time will ensure you are ready to use the equipment safely and effectively during lab.
The Process of Digestion
Digestion begins in the oral cavity, where the mechanical breakdown of food occurs through mastication or chewing. Coordinated movements of the teeth and tongue break food into smaller pieces while saliva mixes with it, forming a bolus, a soft cohesive mass that is lubricated and contains enzymes to aid digestion. The tongue then pushes the bolus into the pharynx, where the pathways for food and air separate.
From the pharynx, the bolus moves down the esophagus through peristalsis, a rhythmic wave of smooth muscle contractions, until it reaches the stomach. Several factors influence peristalsis, including:
- The strength and duration of smooth muscle contractions in the gastrointestinal (GI) tract.
- Neuromuscular signaling between the GI tract and smooth muscle.
- The composition of chyme and hydration levels.
Together, these factors regulate the efficiency of digestion and ensure the smooth transit of food through the digestive system. Throughout this process, the GI system’s regulatory mechanisms continuously optimize digestion and absorption while efficiently eliminating waste.
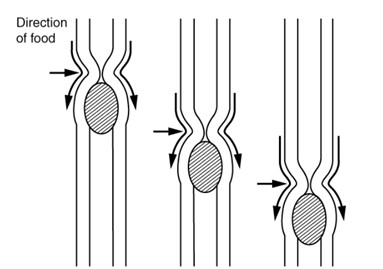
The image illustrates peristalsis in the digestive tract. It shows a section of the tubular digestive organ with its muscular walls. One area of the tube is narrowed due to muscle contraction behind the food bolus, while the section in front of the bolus is relaxed and widened. Arrows indicate the direction of movement, demonstrating how alternating contractions and relaxations create wave-like motions that push food forward.
(Figure by OpenStax, used under a Creative Commons Attribution license.)
The Role of the Stomach and Intestines
The stomach plays a crucial role in digestion by vigorously mixing food with gastric juices, breaking it down into a highly acidic mixture known as chyme. This digested material then enters the small intestine, where nutrient absorption primarily occurs in the duodenum.
Next, the large intestine absorbs excess water, and the remaining waste moves toward the rectum. Finally, the waste is expelled through the anus, which consists of both involuntary and voluntary muscles. The voluntary muscles allow for conscious control over bowel movements, enabling individuals to regulate when they need to use the restroom.
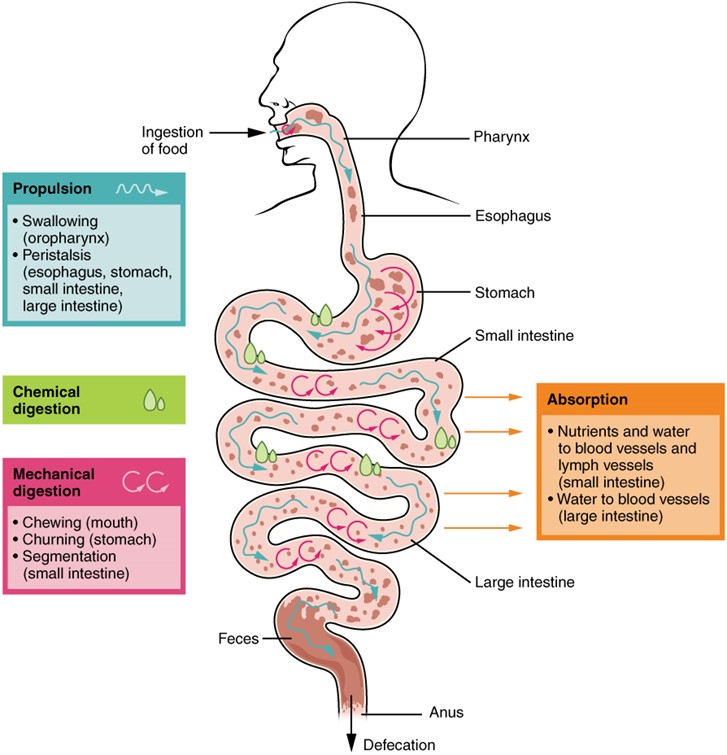
An overview diagram of the digestive processes that occur throughout the gastrointestinal tract. It shows the major organs of the digestive system such as the mouth, esophagus, stomach, small intestine, and large intestine with labels indicating the key processes that take place in each region. The processes typically include ingestion, propulsion, mechanical digestion, chemical digestion, absorption, and defecation. Arrows or labels connect each organ to its associated function, illustrating how food moves through the system while being broken down and absorbed.
(Figure by OpenStax, used under a Creative Commons Attribution license.)
In this lab, you will work in groups to observe the physiological movements involved in swallowing and digestion. Using a stethoscope, you will listen to digestive sounds and calculate the rate of peristalsis. As you collect your data, consider what is causing these movements and discuss with your group the factors such as diet, stress, or hydration that might influence the rate of peristalsis. Think about how the mechanical and chemical processes of digestion are coordinated to move food through the digestive tract.
Questions
-
- How does mechanical digestion differ from chemical digestion? Give examples of each type of digestion.
- What is the optimal pH for pepsin activity?
- What process breaks down proteins into amino acids?
- What is the process by which new glucose molecules are produced from non-carbohydrate sources?
- After enzymatic digestion, lipids are absorbed as fats/fatty acids into __________ of the duodenum.
- What factors can influence the rate of peristalsis?
Adapted from Human Physiology Lab Manual by Jim Blevins, Melaney Farr, and Arleen Sawitzke, Salt Lake Community College.

