4.1 PreLab Reading – Nerve Cells and Electrical Signaling
To comprehend the functioning of electrically active cells, such as neurons and muscles, it is essential to understand the driving forces acting on ions. This knowledge clarifies why specific transport mechanisms are employed for particular substances in distinct locations. A grasp of these concepts is crucial for determining the appropriate IV solutions and understanding the mechanisms of drug action, both of which depend on the transport and diffusion of substances into and out of cells.
Electrochemical Gradient
In lab 3, we explored cell transport and the relationship between primary and secondary active transport. Now, we will focus to the cell as a whole and how it maintains its overall charge. The intracellular compartment has higher concentrations of solutes such amino acids, proteins, ATP, and other phosphate-containing molecules (e.g., DNA and RNA – all phosphate containing molecules have a negative charge), as well as electrolytes like K+. In contrast, the extracellular compartment typically has higher levels of Na+, HCO3-, CL-, Ca2+, and glucose. These concentration differences create an electrochemical gradient between the two compartments. In this lab, we will explore how these electrochemical gradients drive electrical signaling in neurons.
Chemical Force
When a substance moves down its concentration gradient, the reaction releases energy (exergonic) and occurs spontaneously. Conversely, moving a substance against its concentration gradient requires energy input (endergonic) and does not happen spontaneously. The concentration of solutes significantly influences the energetics of a solution, as substances naturally tend to spread out. Therefore, the greater the concentration, the stronger the tendency for diffusion.
The direction of the chemical driving force is always down the substance’s concentration gradient, from higher to lower concentration. You can visualize this gradient as “pushing” particles to spread out. The magnitude of the chemical driving force is proportional to the concentration gradient: the larger the difference in concentration, the greater the energy driving the substance to diffuse.
Electrical Force
Ion = a charge chemical
Cation = a positive charge ion (+)
Anion = a negative charged ion (-)
Voltage refers to the separation of charges. The cell membrane acts as a barrier that separates ions between the ICF and ECF compartments, creating a voltage difference known as membrane potential (Vm). This potential is due to the unequal distribution of cations and ions across the membrane and is measured in millivolts (mV).
- The ICF has slightly more anions than cations, giving a net negative (-) charge.
- The ECF has a slightly more cations than anions, resulting in a net positive (+) charge relative to the ICF.
Since opposite charges attract and like charges repel, this charge separation across the membrane acts as a source of potential energy, similar to a battery. When ions move across the membrane through channels, they generate current, which powers cellular functions.
Resting Membrane Potential and Ion Movement
For most cells, the resting membrane potential (Vm) is approximately -70 mV, meaning the inside of the cell is more negative compared to the outside. This potential is established primarily by the Na+/K+ pump and ion leak channels:
- The Na+/K+ pump actively transports 3 Na+ ions out and 2 K+ ions into the cell, using the energy from ATP hydrolysis. Since more positive charges are pumped out than in, the ECF becomes more positive over time.
- In addition to the pump, leak channels allow passive ion movement. There are more K+ leak channels than Na+ leak channels, allowing more K+ to leave the cell than Na+ enters. This contributes to the negative charge inside the cell.
How Does the Membrane Potential Generate an Electrical Driving Force?
The movement of charged particles follows the same principle as magnets:
- Like charges repel, and opposite charges attract.
- An ion will move toward the side with the opposite charge and away from the side with the same charge.
The magnitude of the electrical driving force depends on:
1) The size of the membrane potential (Vm) – A greater voltage difference leads to a stronger force.
2) The charge of the ion – Higher charges (e.g., Ca2+ vs Na+) experience stronger forces.
Electrochemical Force: The Total Driving Force
Ions are influenced by both:
- Chemical force – driven by concentration gradients.
- Electrical force – driven by membrane potential.
Since forces have both direction and magnitude, the net electrochemical force is the sum of these two forces.
- If the chemical and electrical forces act in the same direction, the ion will move in that direction, with a force equal to their sum.
- If they act in opposite directions, the net movement depends on which force is stronger, determining both the final direction and magnitude of ion movement.
For molecules without charge (e.g., glucose, oxygen), only the chemical force affects their movement, since the electrical force only applies to charged ions.
By understanding these principles, we can determine how ions move across membranes and how cells use electrical signals to communicate and function.
Electrochemical Force: Determining Direction and Magnitude
The electrochemical force is the sum of two forces: chemical force and electrical force. To determine an ion’s movement, we compare its equilibrium potential (Ex, where x is an ion) to the membrane potential (Vm). The equilibrium potential is the voltage at which an ion’s electrical and chemical forces are equal, meaning there is no movement of that ion.
Equilibrium Potential of Key Ions:
ENa+ = +60 mV
EK+ = -94 mV
ECa2+ = +135 mV
ECl- = -88 mV
Determining the direction and magnitude of ion movement:
- Compare the ion’s equilibrium potential to the membrane potential.
- If Vm = Eion, the ion is at equilibrium, and no net force acts on it.
- If Vm is not equal to the Eion, the ion will move in the direction that brings Vm closer to Eion.
- Calculate the magnitude of the force acting on the ion.
- The magnitude is the absolute difference between Vm and Eion.
- Force is always a positive value because it represents the strength of the push or pull on an ion, no its direction. To find the magnitude of the electrochemical force, we simply calculate the difference between the Vm and the Eion and ignore any negative signs. This is why we use the absolute value, it ensures we’re measuring the size of the force, not its direction.
Example: Na+ movement
- The membrane potential is -70 mV.
- The equilibrium potential of Na+ is +60 mV.
- Since Vm is lower than ENa+, Na+ will move into the cell (toward the more positive value).
- Magnitude of the force: ENa+ – Vm = +60 – (-70) = 130 mV
The electrochemical force on Na+ is 130 mV, directed into the cell.
By applying this method to other ions, you can determine their movement across the membrane and the strength of their driving force.
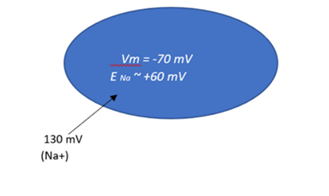
Determining direction and magnitude for Na+: Blue oval represents the ICF. (Electrochemical force direction and magnitude for Na+ by Evelyn Mendoza is used under a Creative Commons Attribution-NonCommercial license.)
The Nervous System
The nervous system consists of two types of cells: neurons and glial cells. Neurons are specialized excitable cells, capable of generating action potential (APs), which are fast, large electrical signals that allow rapid communication throughout the body. In contrast, glial cells, make up the majority of the nervous system, play a crucial roles in supporting neurons in various ways, such as providing structural, metabolic, and functional support. There are four types of glial cells: oligodendrocytes, Schwann cells, astrocytes, and microglia.
Neuronal Structure
Since this lab will be focusing on neuronal function (and not functions of glial cells), you need to understand the basic microanatomy of a neuron. It can be divided into three main functional parts:
- Cell Body (Soma): The cell body contains the nucleus and organelles, carrying out normal metabolic functions. It receives input in the form of neurotransmitters and has numerous ion channels.
- Dendrites: Dendrites are small branches extending from the soma, specialized to receive input in the form of neurotransmitters. They have many ion channels.
- Axon: The axon is a long strand of plasma membrane specialized for transmitting information. Most neurons have one main axon that can branch into collateral axons, allowing information transmission to multiple sites. The axon hillock, where the axon originates from the cell body, is specialized to initiate potentials. The axon terminal at the end of the axon releases neurotransmitters in response to action potentials. They have many ion channels.
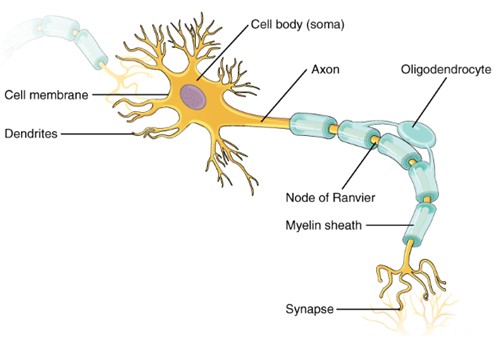
Neuron: Structure of a neuron. (Figure by OpenStax is used under a Creative Commons Attribution license.)
Major Channel Types in Neurons
- Leak Channels (or nongated channels): Found throughout the neuron and always open, maintaining the resting potential.
- Ligand-Gated Channels: Open/close in response to specific ligand (chemical signal) binding (often a neurotransmitter). These channels are dense on the cell body and dendrites.
- Stimulus-gated channels (more to come in the Sensory Lab). Food or odorants act as ligands to open/close ligand-gated channels. But there are also non-chemical stimuli such as mechanical stresses (hearing, balance) and light (vision) when the specific type of energy interacts with specific types of channels to open/close their gates.
- Voltage-Gated Channels: Open/close in response to changes in membrane potential. Voltage-gated Na+ and K+ channels are densely packed on the axon, especially at the hillock. Voltage-gated Ca2+ channels, mainly at the axon terminal, open is response to action potentials and trigger neurotransmitter release.
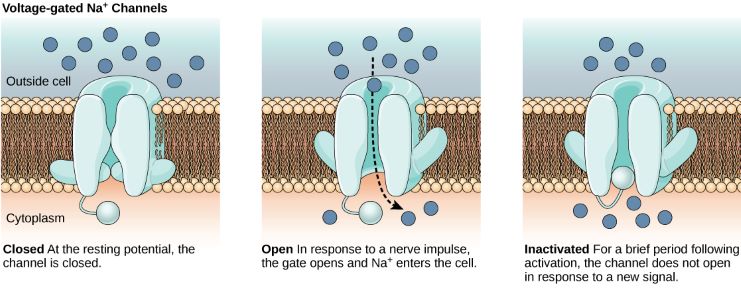 Na+ Voltage-Gated Channel: From left to right: Voltage-gated channel closed; Voltage-gated channel open; Voltage-gated channel inactivated. (Figure by OpenStax is used under a Creative Commons Attribution license.)
Na+ Voltage-Gated Channel: From left to right: Voltage-gated channel closed; Voltage-gated channel open; Voltage-gated channel inactivated. (Figure by OpenStax is used under a Creative Commons Attribution license.)
Neurons are 25 times more permeable to K+ than Na+ due to more K+ leak channels, making the resting membrane potential closer to the K+ equilibrium potential (-94 mV) than the Na+ equilibrium potential (+60 mV). Neither ion reaches equilibrium because the Na+/K+ pump continuously maintains the gradients, ensuring neural stability. At rest, K+ leaks out, and Na+ leaks in, but the Na+/K+ pump compensates for these leaks by actively transporting K+ back in and Na+ out.
Electrical Signaling through Changes in Membrane Potential (Vm)
Gated ion channels open or close in response to stimuli, allowing specific ions to move and change the membrane potential towards the equilibrium potential for that ion. Changes in membrane potential are named based on their direction relative to the resting potential (-70 mV):
Depolarization: Membrane potential become less negative or fully positive.
Hyperpolarization: Membrane potential becomes more negative.
Repolarization: Membrane potential returns to resting state.
There are two main types of electrical signals in neurons due to gated ion channel activity:
- Graded Potentials (GPs):
- Small, short-range, and decrease in size over distance (decremental).
- Often result from neurotransmitter binding to ligand-gated receptors on dendrites.
- Magnitude is proportional to stimulus strength (graded). These potentials can add together (summate) if multiple stimuli occur close enough in time.
- Can be hyperpolarizing (inhibitory) or depolarizing (stimulatory).
- Determine if an action potential will be generated, requiring the graded potential to reach a threshold level.
- Decrease in strength with distance due to current loss through leak channels. Since graded potentials are decremental, the summation helps to ensure that the signal reaches a sufficient strength to trigger an action action potential at the axon hillock if the depolarization reaches the threshold.
- Action Potentials (APs):
- Large, stereotyped (all look the same), can move long distances, and do not decrease in size. The nature of action potentials makes summation impossible. Once an action potential begins, it follows a fixed, unidirectional pathway, and its magnitude is the same, regardless of the stimulus strength.
- All-or-nothing events; they either occur fully or not at all. Action potentials cannot combine with each other in the same way graded potentials can.
- Comprise three phases: depolarization, repolarization, and after-hyperpolarization.
Phases of an Action Potential:
- Depolarization: Membrane potential changes from -70 mV to +30 mV due to Na+ permeability increase.
- Repolarization: Membrane potential returns to -70 mV as Na+ permeability decreases and K+ permeability increases.
- After-Hyperpolarization: Membrane potential briefly becomes more negative than the resting potential due to slow closure of K+ channels.
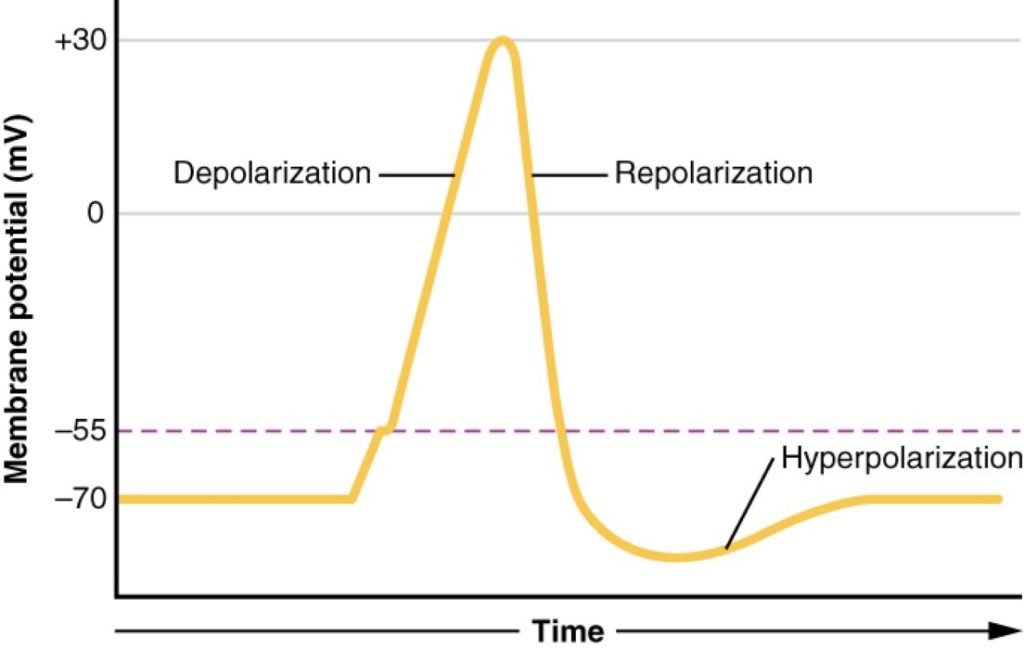
Action Potential: Three phases of action potential: depolarization, repolarization, and hyperpolarization. (Figure by OpenStax is used under a Creative Commons Attribution license.)
Refractory Periods:
The refractory periods seen in neuronal (as some other types) of APs are a result of the Na+ voltage-gated channel having 2 gates and those gates needing to reset in order for another AP to occur. There are two phases of the refractory period:
- Absolute Refractory Period: This period lasts 1-2 ms, during which another AP cannot be generated. The first reason for this is that the positive feedback loop driving the opening of voltage gated Na+ channels continues until their inactivation gates close. The second reason is that the Na+ inactivation gates cannot reset until the membrane potential returns to its resting state. Together, these factors prevent the neuron from firing another AP during this time. As a result, a neuron can fire only 500-1000 APs per second (Hz).
- Relative Refractory Period: This period lasts 5-15 ms, during which a suprathreshold stimulus can generate another AP. During this time, most Na+ inactivation gates are reset and can open again. However, the voltage-gated K+ channels are still open or partially open, meaning the cell is still highly permeable to K+. This causes the membrane potential to remain hyperpolarized for a short time. To generate another AP during this period, a strong stimulus is needed to overcome the hyperpolarized state and drive enough Na+ influx to reach the threshold.
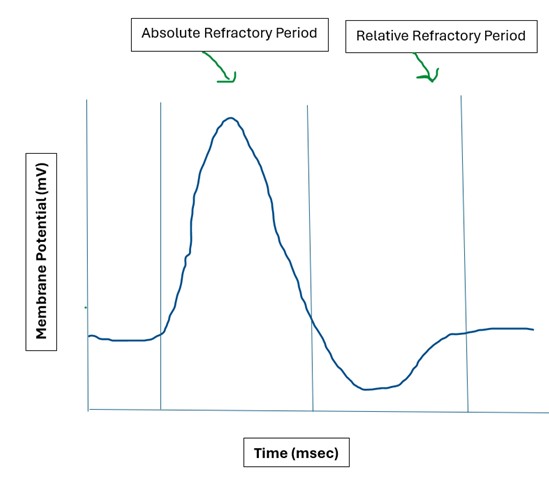
Refractory Periods: Absolute refractory period and relative refractory period.(Refractory periods by Evelyn Mendoza is used under a Creative Commons Attribution-NonCommercial license.)
Questions
-
- Which solutes are found in higher concentrations inside the cell?
- If the concentration of a substance in the ECF is 132mM/L and the concentration of the ICF is 50mM/L, which direction will the chemical force pull the substance?
- Describe the differences in ion concentrations and charges between the intracellular fluid (ICF) and extracellular fluid (ECF).
- What are the equilibrium potentials for the following ions that we will be using in this lab? Be sure to include the correct sign (+/-) before the number.
- Na+
- K+
- Ca2+
- Cl-
- For each of the following types of ion channels, describe the area of the neuron where they are most abundant:
- Leak Channels
- Ligand-gated Channels
- Voltage-gated Channels
Adapted from Human Physiology Lab Manual by Jim Blevins, Melaney Farr, and Arleen Sawitzke, Salt Lake Community College.

