3.1 PreLab Reading – Cell Transport
Cell transport is essential in human physiology because it ensures that cells receive the necessary nutrients, remove waste products, and maintain homeostasis. Key processes driving this exchange include osmosis, diffusion, and active transport. These mechanisms facilitate the movement of substances across the cell membrane, ensuring the proper balance and function of the cell.
Simple Diffusion is the movement of a substance from high concentration area to low concentration area. It is a key mechanism for the movement of gasses within and between different fluid compartments in the body. For example, oxygen and carbon dioxide rely on this type of diffusion to move between interstitial fluid (ISF) and intracellular fluid (ICF).
Osmosis is the diffusion of water from an area of high concentration (and low tonicity) to an area of low water concentration (and high tonicity). Osmosis can be simple; water wiggling its way through the cell membrane directly, or facilitated; using large channel proteins called aquaporins. Figure 1 presents a classic laboratory example that demonstrates osmosis.
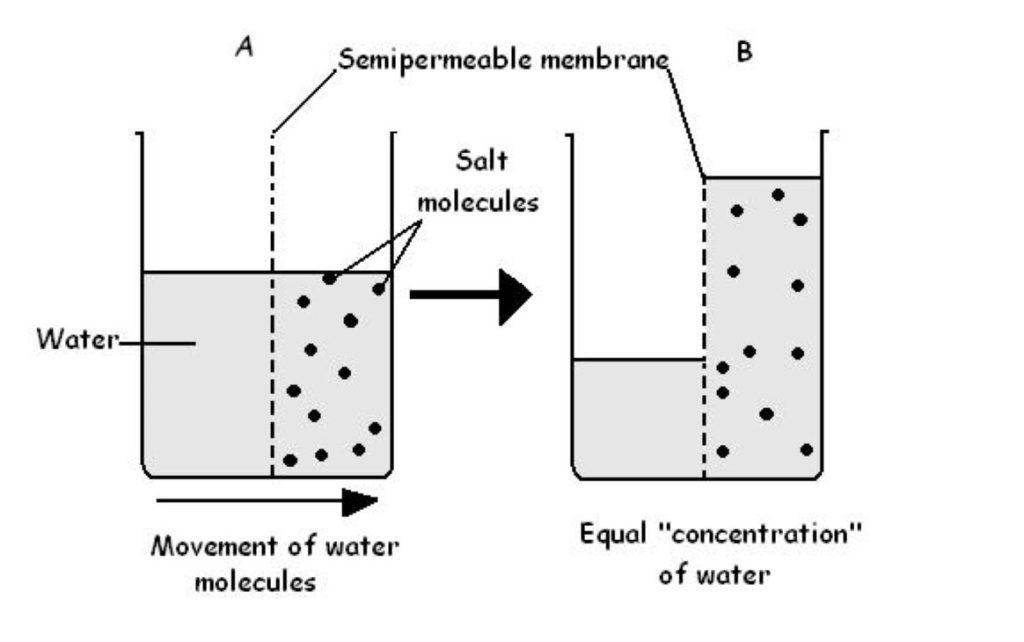
Figure 1: A classical experiment that demonstrates osmosis and osmotic pressure. (Figure by Wikimedia is used under a Creative Commons Attribution license.)
Throughout this laboratory session, you will explore the factors influencing diffusion and osmosis while gaining insight into other models of cellular transportation.
Vocabulary
ICF: Intracellular Fluid – refers to the fluid inside the cell.
ECF: Extracellular Fluid – refers to the fluid outside the cell. This is synonymous with internal environment. This fluid is divided in two main components: plasma, which is the fluid in the blood, and interstitial fluid (ISF), which surrounds the body’s cells outside the blood.
Osmosis: The process of water moving from an area of high concentration to an area of low concentration.
Simple Diffusion: The movement of molecules from areas of high to low concentration across a cell membrane.
Facilitated Diffusion: The transport of molecules (that cannot simply diffuse through a cell membrane) across the cell membrane from areas of high to low concentration facilitated by carrier or channel proteins (transmembrane proteins).
Selectively Permeable: Characterized by the ability to select which molecules will cross. A cellular membrane is selectively permeable.
Semi-permeable: Permeability is based on the size of molecules, such as dialysis tubing.
Permeant Solutes: Solutes capable of crossing the plasma membrane, leading to equilibrium between ICF and ECF. They do not affect the eventual volume of the cell and are irrelevant for measuring tonicity.
Impermeant Solutes: Solutes unable to cross the plasma membrane, thus unable to reach equilibrium between ICF and ECF. They affect the eventual volume of the cell.
Crenate: The process of cell shrinkage due to fluid exiting the cell through osmosis.
Lysis: The bursting open of a cell due to excessive fluid entering the cell through osmosis. The term “hemolysis” is used specifically for red blood cells.
Tonicity: The capacity of solutions to draw water across the membrane, determined solely by the concentration of impermeant solutes.
Isotonic Solution: A solution with the same concentration of impermeant solutes as the ICF, resulting in no net movement of water between ECF and ICF.
Hypotonic Solution: A solution with a lower concentration of impermeant solutes than the ICF, leading to the movement of water from ECF to ICF.
Hypertonic Solution: A solution with a high concentration of impermeant solutes than the ICF, causing water to move from the ICF to the ECF.
Transport in Human Physiology
The cell membrane is important for regulating the movement of substances in and out of the cell. Some molecules can easily pass through membrane, while others cannot. Later in this discussion, we’ll explore which substances can or cannot cross the membrane. It is important to note that for some molecules, cell does not have direct control over the movement of these substances across its membrane.
Molecules that can pass through the membrane without assistance use a process known as simple diffusion. This involves the movement of molecules across the cell membrane along their concentration gradient (from high concentration area to low concentration area). These substances lead a “simple life”, freely entering or exiting the cell as needed, without requiring any help.
Substances that cannot pass the membrane via simple diffusion need assistance from specialized proteins, such as carriers or channels proteins. Some of these substances move along their concentration gradient, while others move against it. Those moving with their concentration gradient require minimal help and undergo facilitated diffusion. The term “facilitated” highlights the support provided in the process, while “diffusion” refers to movement along the concentration gradient. Table 1 summarizes the differences in composition between the intracellular fluid (ICF) and extracellular fluid (ECF).
| SOLUTE | ICF | ECF |
| K+ | high | low |
| Na+ | low | high |
| Mg2+ | low | high |
| Ca2+ | low | high |
| Cl– | low | high |
| HCO3 – | low | high |
| Pi | high | low |
| Amino Acids | high | low |
| Glucose | low | high |
| ATP | high | low |
| Protein | high | low |
Table 1: Concentrations of selected solutes in the intracellular fluid and extracellular fluid.
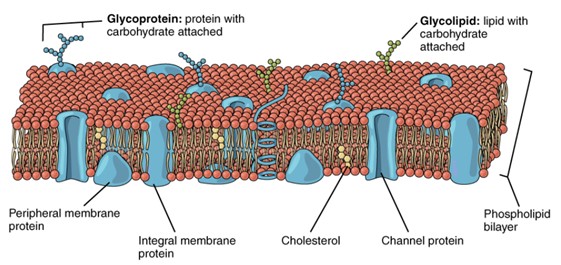
Phospholipid Bilayer: Structure of the cell membrane. (Figure by OpenStax is used under a Creative Commons Attribution license.)
On the other hand, substances moving against their concentration gradient, commonly referred to as “pumping,” require more assistance than those involved in facilitated diffusion. These substances depend on active transport, which utilizes carrier proteins. Later, we will explore the types of active transport in greater detail.
Now you should remember/understand the differences and similarities between osmosis, simple, and facilitated diffusion. To really understand active transport and how that differs from diffusion and osmosis, you will need to pay special attention to the following categories:
Things that Cannot move on their own through the cell membrane:
These substances are generally hydrophilic and/or too large to slip between the phospholipid bilayer. This list includes proteins/peptides (and amino acids), carbohydrates (and simple sugars), nucleic acids (and nucleotides), all ions (such as Na+, K+, Ca2+, Cl–, H+, etc.), and triglycerides (even though these are lipids they are way too big to cross the membrane).
Note: In certain circumstances, such as high temperature (which increases the rate of diffusion), very small substances such as simple sugars like glucose and fructose (and very small amino acids) can slip through the cell membranes in small numbers (between gaps between the phospholipids as they move). However, most of these substances do not move through cell membranes on their own, so this small amount of simple diffusion does not usually have any significant contribution to the movement of these particular substances.
Things that Can move on their own through the cell membrane:
These substances are generally hydrophobic and/or small enough to slip between the phospholipids. This list includes gasses (such as O2 and CO2), steroids, carotenoids (such as vitamin A), eicosanoids (such as thromboxane), alcohols (such as ethanol and propanol). The larger an alcohol molecule is, the more lipid-soluble it is and can “slip” through the cell membrane easier, and water (this is the weird one since it is clearly NOT hydrophobic, but it is very small. It makes up 2/3 of every cell so there is a LOT of it). Some water crosses the cell membrane by simple diffusion, but most of water diffuses across cell membranes through aquaporins (water channels).
The Two Types of Active Transport
1) Primary Active Transport (also called direct active transport). This type of transport uses the cell’s PRIMARY energy source – ATP – as the energy source to force a substance, usually an ion or ions, against its/their concentration gradient using a protein (pump). It is called direct active transport because ATP is used DIRECTLY at the pump itself.
2) Secondary Active Transport (also called indirect active transport). This type of transport uses a SECONDARY form of energy – a concentration gradient – to move a substance (usually sugars, amino acids, and vitamins) against the concentration gradient using a carrier protein. It is called INDIRECT because it indirectly uses energy (concentration gradient or electrochemical as a source of energy).
A key example of primary active transport is the Sodium-Potassium Pump, which plays an important role in human cell membranes by maintaining the proper balance of sodium and potassium ions across the membrane.
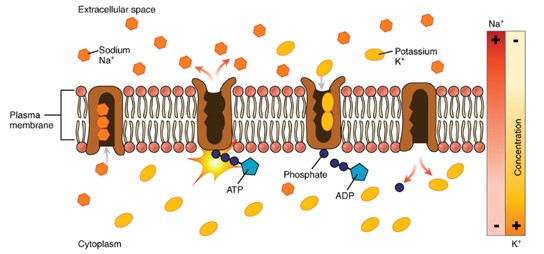
Active Transport: Primary active transport, Na+/K+ pump (Figure by OpenStax is used under a Creative Commons Attribution license.)
The Role of Primary/Direct Active Transport
What purpose does primary/direct active transport serve? It creates an ion gradient! While this may seem like a redundant process, it is essential because the cell relies on the ionic gradient established by primary active transport to power secondary active transport.
How Primary and Secondary Active Transports Work Together
Voltage is the separation of charges between two different locations. Current is what happens when charged particles move. We use current to power lights, heaters, etc.
In cells, charges are created by the ions that reside inside/outside of the cell. Ions, no matter how small they are cannot move through a cell’s membrane without the help of a transport protein due to their +/- charge. Voltage is a measurement of the differences in the amounts/types of charged particles between two locations. Your cell’s membrane effectively separates charges into two very distinct locations: the intracellular (intra- means “within”) compartment (full of intracellular fluid – ICF) and the extracellular (extra – means “outside of or not part of”) compartment (full of extracellular fluid – ECF).
Primary active transporters use the primary energy source, ATP, to move ions against their gradients. Why? Because they are moving charges in a way to create a charge separation across the membrane = voltage. Think of the Na+/K+ pump. It forces 3 Na+ out and 2 K+ in (using 1 ATP to do it), and over time it becomes more positive (+) outside compared to inside the cell (which is, in comparison negative because it is full of things like DNA and RNA, which have negatively charged phosphate groups). Cells use these primary active transporters to make the voltage across the membrane. The voltage across cell membranes is called the membrane potential (Vm). The Vm has the potential to do work, hence the term “membrane potential”.
Secondary active transporters USE this voltage difference between the ECF and ICF to create a current (which is defined as moving charges). An example of this type of active transport is the Na+/glucose symporter. This specific transporter uses the current of Na+ moving down its gradient to power glucose being forced against its gradient. The current from Na+ moving is used as the energy source to pump glucose against its concentration gradient! Therefore, secondary active transporters use a secondary form of energy: voltage, the concentration gradient of ions across the membrane – membrane potential (Vm). However, they indirectly use ATP because without the primary active transporters (which use ATP) there would be NO energy (voltage: the separation of charges across the membrane) for secondary active transporters to use!
Ions are atoms/molecules that have gained or lost electrons to stabilize their valence shells, so they have chemical concentration gradients. But ions also have charges, and physical laws dictate that like charges (+ and + or – and -) repel each other, and opposite charges (+ and -) attract each other. Therefore, ions also have another force, the electrical gradient force, that acts upon them. The result of both the chemical gradient and electrical gradient acting upon an ion is the resulting force called the electrochemical gradient.
Note: Understanding the electrochemical gradient is essential, as it governs not only transport but also the function of excitable cell types like neurons and muscles. We will explore this concept in more depth in Lab 4: Nerve Cells and Electrical Signaling.
Distinguishing Membrane Proteins
Membrane proteins, including channels, carriers, and pumps, are essential for the movement of molecules across the membrane. Let’s explore the unique functions and characteristics of each type:
Channels: These proteins form a narrow pathway that allow specific ions or water to pass through the membrane using this tunnel-like protein. The channel protein is very specific in what type of ion it allows to go through it. The ions moving through channels can only move with their gradients: from high gradient to low gradient – facilitated diffusion. There are different types of channels:
- Leak Channels: these are always open to their specific ion.
- Ligand-gated Channels: these are open in response to the binding of a specific signaling molecule (ligand).
- Voltage-gated Channels: these are open or close in response to changes in Vm. More on these in Lab 4.
Carriers: are protein transporters that change their shape to move a substance across the membrane. Carriers are very specific and can either transport a substance down its concentration gradient (facilitated diffusion) or against its gradient (active transport). When a molecule binds to a carrier, the carrier undergoes a conformational change to facilitate the molecule’s passage. This binding step limits the rate of transport, as carriers can become saturated once all available binding sites are occupied. As a result, carrier-mediated transport is slower than simple diffusion or facilitated diffusion through channels. Larger molecules like amino acids and glucose often rely on carriers for facilitated diffusion, as they are too large for simple diffusion or channel-mediated transport. 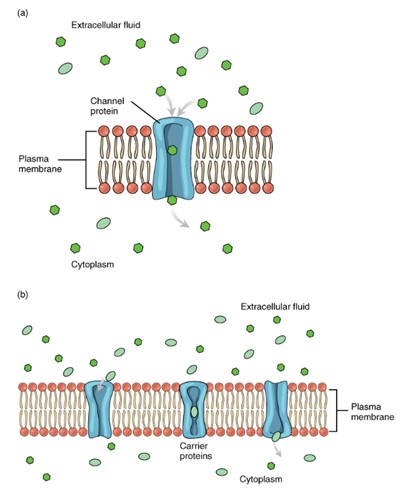
Transmembrane Protein: Carrier protein. (Figure by OpenStax is used under a Creative Commons Attribution license.)
Pumps: When carrier proteins transport molecules against their concentration gradients, they are referred to as pumps. Similarly to how lifting weights or pumping water requires energy, pumps also require energy to function. This energy expenditure is essential for maintaining concentration gradients across the cell membrane. Pumps can be either primary or secondary active transporters.
| Membrane Protein | Direction of Molecules | Type of Transport |
| Channel | Only down/with concentration gradient | Facilitated Diffusion
|
| Carrier | Down or against concentration gradient | Facilitated diffusion or active transport (pump) |
These distinctions highlight the diverse roles and mechanisms employed by membrane proteins in cellular transport.
Bulk Transport
Cells use membrane-bound vesicles to move large quantities of substances in or out of themselves at once, a process requiring energy. This energy drives motor proteins like kinesin and dynein which change shape using ATP to propel vesicles. Bulk Transport can only be used to move substances that cannot move through the membrane on their own (they cannot use simple diffusion or osmosis). Bulk transport includes two main processes:
- Exocytosis: The prefix “Exo” (out), combined with “cyto” (cell) and “osis” (process of), signifies moving substances out of the cell in bulk. Through exocytosis, cells expel large amounts of molecules, such as waste or hormones, using vesicles that fuse with the cell membrane, increasing its surface area. Can you think about why this happens?
- Endocytosis: The prefix “Endo” (inside), along with “cyto” and “osis”, indicates bringing substances into the cell. In endocytosis, vesicles form by budding off the cell membrane and moving inward, which decreases the cell membrane’s surface area. There are three main types of endocytosis.
- Phagocytosis (cell eating): Used by immune cells like macrophages and neutrophils, this process engulfs large particles such as bacteria, forming a large vesicle around the particle for ingestion.
- Pinocytosis (cell drinking): In this process, cells capture droplets of fluid filled with small molecules, which are then enclosed in small vesicles. These vesicles later release their contents into the cell’s cytoplasm.
- Receptor-Mediated Endocytosis (RME): Here, a specific receptor binds to target molecules to bring them into the cell. For example, liver cells use LDL receptors to internalize cholesterol.
Questions
-
- How does the concentration of different types of permeant and impermeant solutes influence tonicity and the movement of water across the plasma membrane?
- How do different types of transport mechanisms facilitate the movement of substances across the cell membrane (list and explain), and what role do concentration gradients play in determining whether energy or assistance is needed for this process?
- Which membrane protein does not change its overall shape in order to transport molecules?
- What factors determine whether a substance can move across the cell membrane on its own, and which substances are able to do so without assistance?
- How do primary and secondary active transport mechanisms differ in terms of their use of ATP to move substances across the cell membrane?
- How does primary active transport contribute to the creation of a concentration gradient across the cell membrane, and how does this gradient drive secondary active transport?
- Describe the different types of bulk transport and provide an example of each. How do each of these processes function to move substances into or out of cells?
Adapted from Human Physiology Lab Manual by Jim Blevins, Melaney Farr, and Arleen Sawitzke, Salt Lake Community College.

