7 Lab Protocol: Chemistry & pH
Exercise 1: Draw a Bohr Model for a Carbon-13 atom
Read and discuss the pre-activity questions on your data sheet with your group. Complete answering all questions before moving to the next activity.
1. Carbon has an atomic number of six (6). This means all atoms with 6 protons in the nucleus are carbon atoms. How many protons are present in one Carbon-13 atom?
2. Isotopes are different versions of the same element. Two atoms are isotopes of one another if they contain the same number of protons but have different numbers of neutrons. Therefore, different isotopes of the same element will have a different atomic mass = # of protons + # of neutrons (a proton and a neutron are equal in atomic mass). Carbon-13 refers to a carbon atom with an atomic mass of 13. How many neutrons are in one Carbon-13 atom?
3. When thinking about an atom, we will assume that it is uncharged unless we are told otherwise. To be uncharged, the carbon-13 atom must have the same number of electrons as it has protons. How many electrons will be part of the uncharged Carbon-13 atom?
How many electrons will be in the first shell of a Carbon-13 atom?
How many electrons will be in the second shell?
How many electrons will be in the third shell?
4. How many bonds is a Carbon-13 atom expected to make?
5. Draw a Bohr model for Carbon-13 atom. Each group member is to draw their own Bohr model and initial next to it. Include the nucleus and electron shells making sure that the number of protons, neutrons, and electrons are correct and can be clearly counted by the instructor.
Exercise 2: Determine the type of chemical bond
In this activity, you will practice identifying what type of chemical bond two atoms are likely to make depending on the difference in electronegativity between them. Complete the activity labeled Exercise 2 on your data sheet with your group.
| Electronegativity difference between atoms | Type of bond between atoms |
| < 0.4 | Nonpolar covalent |
| 0.4 – 1.6 | Polar covalent |
| > 1.7 | Ionic bond |
Complete the following table in your data sheet.
| Element | # of protons | # of electrons | # of valence electrons | electronegativity |
| Hydrogen | 2.2 | |||
| Oxygen | 3.5 | |||
| Fluorine | 4.1 | |||
| Lithium | 1.0 | |||
| Carbon | 2.5 | |||
| Nitrogen | 3.1 | |||
| Sulfur | 2.4 | |||
| Magnesium | 1.2 |
Complete the following table in your data sheet.
| Element pairs | Electronegativity difference | Type of bond |
| Hydrogen and Oxygen | ||
| Carbon and Hydrogen | ||
| Oxygen and Carbon |
Exercise 3: Identify the model elements by color
Now, you will work with the chemistry models provided in lab. The balls in the chemistry model kit represent different elements and the connectors represent covalent bonds. Based on the number of holes per ball (which represent unpaired electrons and the potential to make a bond), indicate which color of ball represents each of the following elements:
Phosphorus:
Carbon:
Hydrogen:
Nitrogen:
Oxygen:
In this model kit, the pieces used to represent single covalent bonds are short and rigid. The pieces used to represent double covalent bonds are long so that they can bend a little bit. Find examples of each in the kit.
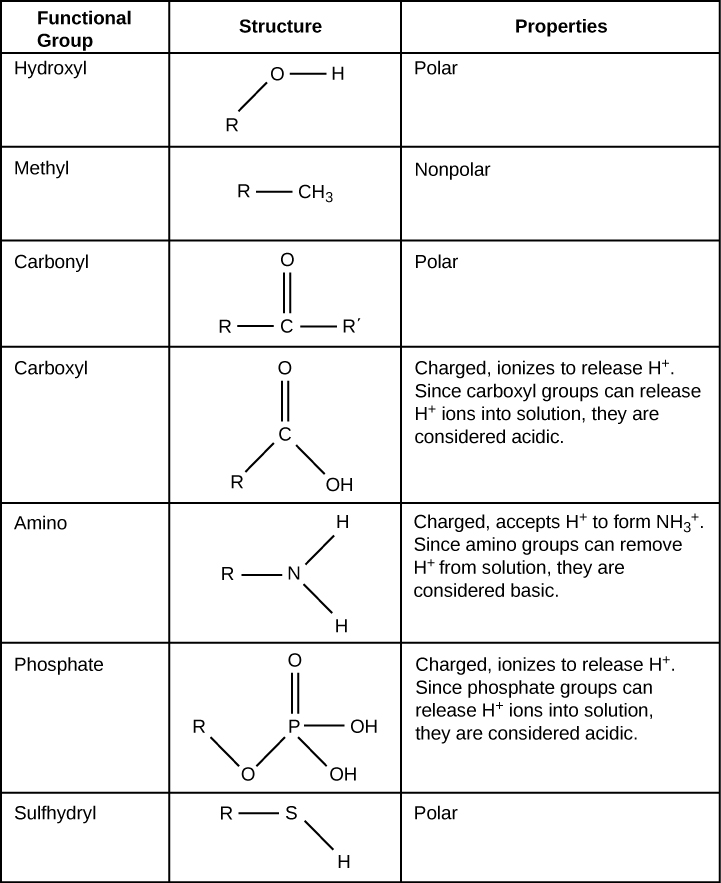
Exercise 4: Build functional groups
Build each of the functional groups depicted in the table below using the correct balls and connectors. Then take a photograph of the models and upload it to Canvas. Do not include a ball to represent the R group (rest of the molecule); however, include a connector to show how the functional group would attach to the rest of the molecule. Note that the yellow ball which represents sulfur has six holes. Sometimes sulfur makes six bonds and sometimes only two.
- Methyl
- Hydroxyl
- Carboxyl
- Carbonyl
- Amino
- Phosphate
Below is a table that displays the functional groups to help you accomplish this task. Note that the colors may be different than the model. Focus on the elements used.
Exercise 5: Build an amino acid using functional groups.
There are four components that make up an amino acid:
- A central carbon, including a covalent bond to one hydrogen.
- An amino group.
- A carboxyl group.
- An R group
Using the functional groups you built in the previous task and other model parts. Build one of the following amino acids: valine, isoleucine, serine, or asparagine (see lab manual for molecular structures). Different groups will build different amino acids so you can compare them.
As a group of two, build one amino acid.
1) Place the amino group that you built in the previous task in front of you.
2) Place the carboxyl group that you built in the previous task in front of you.
3) Attach a connector to a hydrogen atom.
4) Build the R group and include a connector with which you will connect it to the central carbon atom (in blue on diagram).
5) Use a carbon atom with four empty holes. Attach items 1 – 4 to the carbon atom. Your amino acid is complete.
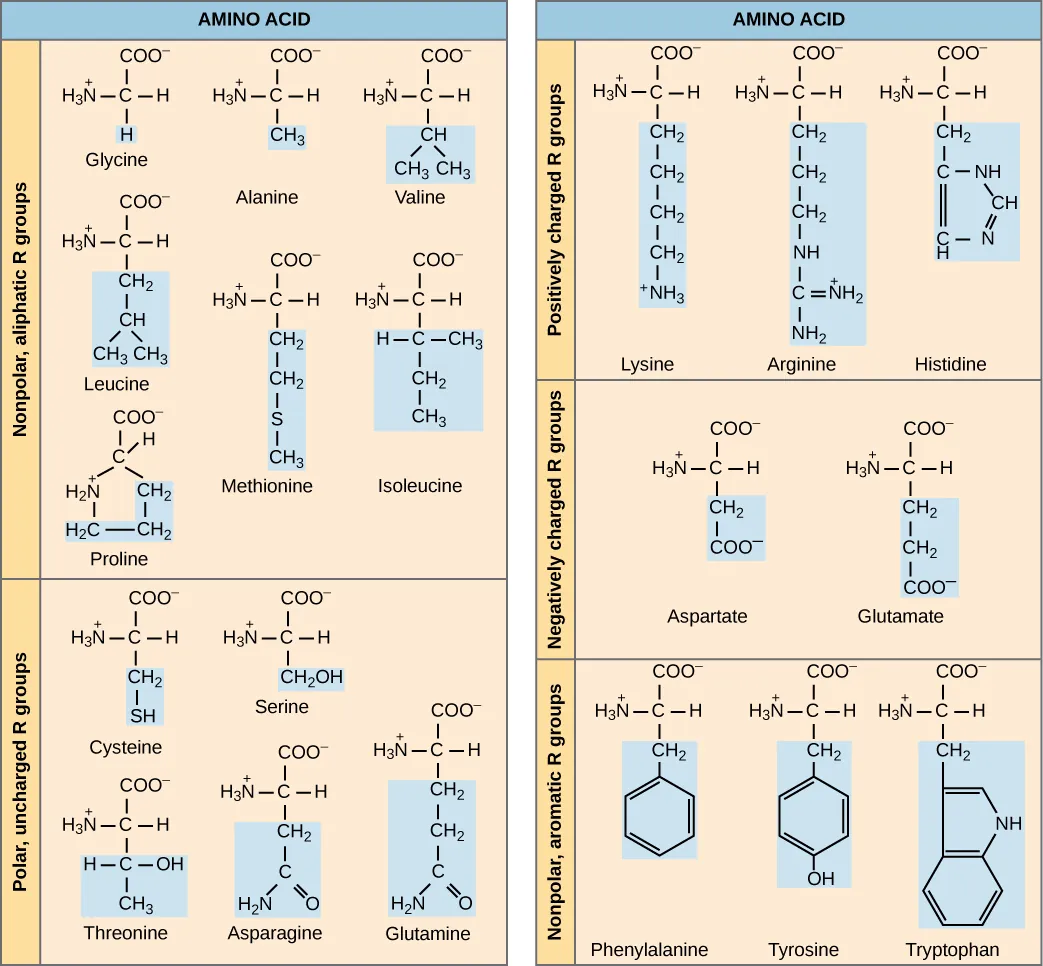
There are 20 common amino acids commonly found in proteins, each with a different R group (variant group) that determines its chemical nature. (Figure by OpenStax is used under a Creative Commons Attribution license.)
Exercise 6: Build two hydrocarbons
Build each of the following hydrocarbons. Each pair of students at each table will build one:
- Chain with 6 carbons and 12 hydrogens (C6H12) including one double bond between any two carbon atoms
- Ring with 6 carbons and 12 hydrogens (C6H12)
Exercise 7: Demonstrate hydrogen bonding
Build five water molecules (H2O).
Lay the water molecules on the table. Line up the water molecules so that the oxygen of one water molecule is pointing toward one of the hydrogens of the other water molecule. Take a picture. Get instructor initials.
What is the name of the attraction between the oxygen of one water molecule and the hydrogen of the other?
Is this a type of covalent bond? Circle one: Yes No
What type of covalent bond is this?
Exercise 8: Evaluating a solution for its buffering capacity
First assess the effect of adding HCl to a known buffer (phosphate buffer, pH = 6) and water (negative control).
- Measure 3 mL of phosphate buffer (known buffer) into one test tube and 3 mL of water (negative control) into another test tube.
- Measure and record the pH of each solution.
- Add 100 μL of 0.1 M HCl to each tube, mix gently by shaking.
- Measure and record the pH of each solution.
Based on molecular formulas and reading labels of alka seltzer and other acid indigestion product, predict which of the solutions below will function as buffers. Indicate why you made each prediction.
Now evaluate the buffering capacity of a variety of liquids in the same way as described above.
- Milk
- 0.1 M NaCl
- Alka Seltzer
- Another product to reduce acid indigestion.
Record pH readings on the data table and indicate if the solution is an effective buffer,
| pH before HCl | pH after HCl | Effective buffer | |
| Phosphate buffer | yes | ||
| water | no | ||
| milk | |||
| 0.1 M NaCl | |||
| Alka Seltzer | |||
| Second product |

