12 Background: Diffusion and Osmosis
Passive Transport
Plasma membranes must allow certain substances to enter and leave a cell, and prevent some harmful materials from entering and some essential materials from leaving. In other words, plasma membranes are selectively permeable—they allow some substances to pass through, but not others. If they were to lose this selectivity, the cell would no longer be able to sustain itself, and it would be destroyed.
Some cells require larger amounts of specific substances. They must have a way of obtaining these materials from extracellular fluids. This may happen passively, as certain materials move back and forth, or the cell may have special mechanisms that facilitate transport.
The most direct forms of membrane transport are passive. Passive transport is a naturally occurring phenomenon and does not require the cell to exert any of its energy to accomplish the movement. In passive transport, substances move from an area of higher concentration to an area of lower concentration. A concentration gradient can be defined as a physical space in which different regions of that space have a different concentration of a particular substance.
Selective Permeability
Recall that plasma membranes are amphiphilic: They have hydrophilic and hydrophobic regions. This characteristic helps move some materials through the membrane and hinders the movement of others. Non-polar and lipid-soluble substances with a low molecular weight can easily slip through the membrane’s hydrophobic lipid core. Substances such as the fat-soluble vitamins A, D, E, and K readily pass through the plasma membranes in the digestive tract and other tissues. Fat-soluble drugs and hormones also gain easy entry into cells and readily transport themselves into the body’s tissues and organs. Oxygen and carbon dioxide molecules have no charge and pass through membranes by simple diffusion.
Polar substances present problems for the membrane. While some polar molecules connect easily with the cell’s outside, they cannot readily pass through the plasma membrane’s lipid core. Additionally, while small ions are small enough to pass through the membrane, their charge prevents them from doing so. Ions such as sodium, potassium, calcium, and chloride must have special means of penetrating plasma membranes. Simple sugars and amino acids also need the help of various transmembrane proteins to be transported across plasma membranes.
Diffusion
Diffusion is a passive process of transport. A single substance moves from a high concentration to a low concentration area until the concentration is equal across a space. You are familiar with diffusion of substances through the air. For example, think about someone opening a bottle of perfume in a room filled with people. The perfume molecules are at their highest concentration in the bottle. Their lowest concentration is at the room’s edges. The molecules will diffuse, or spread away, from the bottle, and gradually, increasingly more people will smell the perfume as it spreads. Materials move within the cell’s cytosol by diffusion, and certain materials move through the plasma membrane by diffusion.
Diffusion expends no energy. On the contrary, concentration gradients are a form of potential energy. The energy is released as substances move down their concentration gradient.
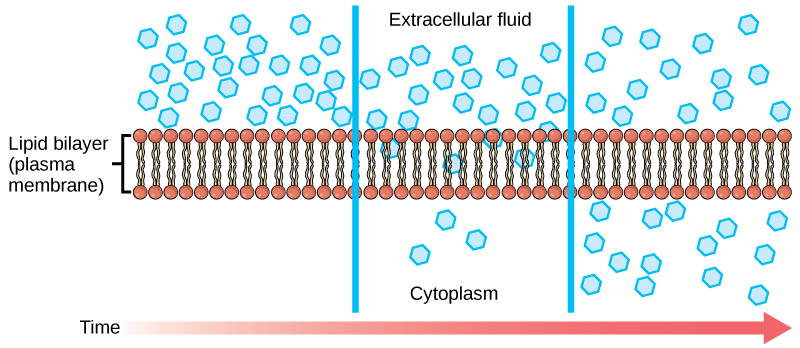
Each separate substance in a medium, such as the extracellular fluid, has its own concentration gradient, independent of other materials’ concentration gradients. Each substance will diffuse according to that gradient. Within a system, there will be different diffusion rates of various substances in the medium.
Factors That Affect Diffusion
Molecules move constantly in a random manner, at a rate that depends on their mass, their environment, and the amount of thermal energy they possess, which in turn is a function of temperature. This movement accounts for molecule diffusion through whatever medium in which they are localized. A substance moves into any space available to it until it evenly distributes itself throughout. After a substance has diffused completely through a space, removing its concentration gradient, molecules will still move around in the space, but there will be no net movement of the number of molecules from one area to another. We call this lack of a concentration gradient in which the substance has no net movement dynamic equilibrium. While diffusion will go forward in the presence of a substance’s concentration gradient, several factors affect the diffusion rate, including:
- Extent of the concentration gradient: The greater the difference in concentration, the more rapid the diffusion.
- Mass of the molecules diffusing: Heavier molecules diffuse more slowly.
- Temperature: Higher temperatures increase molecular movement and therefore the diffusion rate.
- Solvent density: As the density of a solvent increases, the diffusion rate decreases.
- Solubility: Nonpolar or lipid-soluble materials pass through plasma membranes more easily than polar materials, allowing a faster diffusion rate.
- Surface area and plasma membrane thickness: Increased surface area increases the diffusion rate; whereas, a thicker membrane reduces it.
- Distance travelled: The greater the distance that a substance must travel, the slower the diffusion rate.

