8 Background: Biologically Important Molecules
Before investigating the presence of these macromolecules in food, it is important to first perform what is called a positive control and negative control standards test for each macromolecule. A positive control is a sample in an experiment that is treated in a way that is known to give a positive result. Your positive control sample when testing for protein will be egg white, which is known to contain the protein albumin. A negative control is a sample that is known to give a negative result – in these experiments, distilled water will be a good negative control because it is known not to contain any biomolecules. These samples will provide you with a reference for identifying each of the representative macromolecules in your food samples.
Biological macromolecules are large molecules, necessary for life, that are built from smaller organic molecules. The four most important classes of biological macromolecule classes are carbohydrates, lipids, proteins, and nucleic acids. These macromolecules are important cell components that perform a wide array of functions. Combined, these molecules make up the majority of a cell’s dry mass (recall that water makes up the majority of its complete mass). Biological macromolecules are organic, meaning they contain carbon. In addition, they may contain hydrogen, oxygen, nitrogen, and additional minor elements.
Dehydration Synthesis
Most macromolecules are made from single subunits, or building blocks, called monomers. The monomers combine with each other using covalent bonds to form larger molecules known as polymers. In doing so, monomers release water molecules as byproducts. This type of reaction is dehydration synthesis, which means “to put together while losing water.”
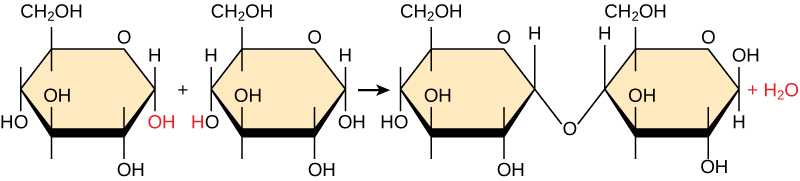
In a dehydration synthesis reaction, a hydrogen from one monomer combines with a hydroxyl group of another monomer, releasing a water molecule. At the same time, the monomers share electrons and form covalent bonds. As additional monomers join, this chain of repeating monomers forms a polymer. Different monomer types can combine in many configurations, giving rise to a diverse group of macromolecules. Even one kind of monomer can combine in a variety of ways to form different polymers. For example, glucose monomers are the constituents of starch, glycogen, and cellulose.
Hydrolysis
Polymers break down into monomers during hydrolysis. A chemical reaction occurs when inserting a water molecule across the bond. Breaking a covalent bond with this water molecule in the compound achieves this. During these reactions, the polymer breaks into two components: one part gains a hydrogen atom (H) and the other gains a hydroxyl group (OH) from a split water molecule.
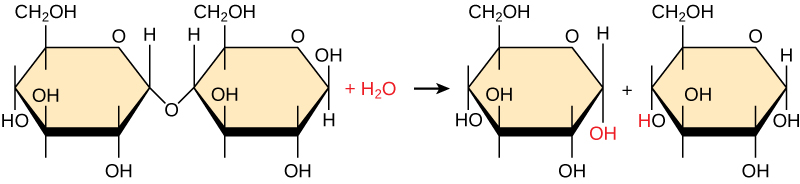
Dehydration and hydrolysis reactions are catalyzed, or “sped up,” by specific enzymes; dehydration reactions increase the length of polymer chains, requiring energy, while hydrolysis reactions decrease their length and release energy. These reactions are similar for most macromolecules, but each monomer and polymer reaction is specific for its class. For example, catalytic enzymes in the digestive system hydrolyze or break down the food we ingest into smaller molecules. This allows cells in our body to easily absorb nutrients in the intestine. A specific enzyme helps break down each macromolecule. For instance, amylase, sucrase, lactase, and maltase contribute to the breakdown of specific carbohydrates. Enzymes called proteases, such as pepsin and peptidase, and hydrochloric acid break down proteins. Lipases break down lipids. As these macromolecules are broken down, energy is released to enable cellular activities.

